J Antimicrob Chemother 2016; 71 Suppl 1: i63–i69 doi:10.1093/jac/dkw068
Y. Feshchenko1, A. Dzyublik1, T. Pertseva2, E. Bratus3, Y. Dzyublik1, G. Gladka4, I. Morrissey5 and D. Torumkuney6*
1State Organization National Institute of Phthisiology and Pulmonology named after F.G. Yanovsky, National Academy of Medical Sciences of Ukraine, 10 Amosova Str., 03680, Kiev, Ukraine; 2Dnepropetrovsk State Medical Academy, 4 Dzovtneva Square 49027, Dnepropetrovsk, Ukraine; 3Dnepropetrovsk State Medical Academy, Diagnostic Center, 4 Dzovtneva Square 49027, Dnepropetrovsk, Ukraine; 4GlaxoSmithKline Ukraine, Pavla Tychyny Avenue, 1-V, 02152, Kiev, Ukraine; 5IHMA Europe Sa` rl, 9A route de la Corniche, Epalinges 1066, Switzerland; 6GlaxoSmithKline, 980 Great West Road, Brentford, Middlesex TW8 9GS, UK
Objectives: To determine the antibiotic susceptibility of respiratory isolates of Streptococcus pneumoniae and Haemophilus influenzae collected in 2011–13 from Ukraine.
Methods: MICs were determined by CLSI broth microdilution and susceptibility was assessed using CLSI, EUCAST and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints.
Results: A total of 134 isolates of S. pneumoniae and 67 of H. influenzae were collected from eight sites in Ukraine. Overall, 87.3% of S. pneumoniae were penicillin susceptible by CLSI oral breakpoints and 99.3% by CLSI iv breakpoints. Susceptibility to amoxicillin/clavulanic acid (amoxicillin), ceftriaxone and levofloxacin was 100% by CLSI and PK/PD breakpoints. Cephalosporin and macrolide susceptibility was ≥95.5% and 88.1%, respectively using CLSI breakpoints. Trimethoprim/sulfamethoxazolewas essentially inactive against pneumococci. Of the 67 H. influenzae tested, 4.5% were b-lactamase positive and all H. influenzae were fully susceptible to amoxicillin/clavulanic acid, ceftriaxone, ciprofloxacin, cefixime and levofloxacin (all breakpoints). Cefuroxime susceptibility was 100% by CLSI but 73.1% by EUCAST and PK/PD breakpoints. A discrepancy was found in macrolide susceptibility between CLSI (∼100% susceptible), EUCAST (22%–43% susceptible) and PK/PD (0%–22% susceptible) breakpoints. Trimethoprim/sulfamethoxazole was poorly active (59.7% susceptible).
Conclusions: Generally, antibiotic resistance was low in respiratory pathogens from Ukraine. However, only amoxicillin/clavulanic acid (amoxicillin), ceftriaxone and levofloxacin were fully active against both species. Trimethoprim/sulfamethoxazole was the least active, particularly against S. pneumoniae. Some susceptibility differences were apparent between CLSI, EUCAST and PK/PD breakpoints, especially with macrolides against H. influenzae. These data suggest that further efforts are required to harmonize these international breakpoints. Future studies are warranted to monitor continued low resistance levels in Ukraine compared with other parts of Eastern Europe.
Community-acquired pneumonia (CAP) and other lower respiratory tract infections (LRTIs) are the second most frequent cause of all-age premature deaths worldwide.1 CAP and other LRTIs are also a major cause of morbidity among children aged <5 years, causing 12 million hospital admissions per annum2 and 1.6 million deaths in young children3 worldwide. CAP also imposes a substantial disease burden on older children4 and adults.5,6 Improving the therapeutic management of pneumonia is one of the top priorities, as defined by international experts, to decrease the CAP burden and improve health worldwide.7
Clinical diagnosis of CAP can be problematic, especially in patients with underlying pulmonary conditions.8 This diagnosis may be influenced by available microbiological testing methods or impeded by antibiotic intake prior to testing.9 Streptococcus pneumoniae and Haemophilus influenzae are the main bacterial pathogens associated with community-acquired respiratory tract infections.10 Antimicrobial resistance in these pathogens is common and resistance surveillance plays an important role in the reporting and management of LRTI. One such study is the ongoing Survey of Antibiotic Resistance (SOAR), which is an antimicrobial resistance surveillance study of key respiratory pathogens conducted in the Middle East, Africa, Latin America, Asia-Pacific and Commonwealth of Independent States countries and has been running since 2002. Herewe report recent data from SOAR for major respiratory tract pathogens collected from hospital sites in the Ukraine.
The following eight centres took part in the study: National Institute of Tuberculosis and Pulmonology, Kiev, Ukraine; Regional Pediatric Clinic, Kiev, Ukraine; Dnepropetrovsk State Medical Academy, Dnepropetrovsk, Ukraine; Vinnitsa State Pirogov Memorial Medical University, Vinnitsa, Ukraine; Regional Pediatric Clinic, Lviv, Ukraine; Regional Hospital, Ivano-Frankivsk Ukraine; Regional Hospital, Simferopol, Ukraine; and Regional Children's Hospital, Zaporozhye, Ukraine.
Isolates were collected (from community-acquired infections/outpatients/university hospitals) during 2011–13 from a variety of sources, including bronchoalveolar lavage, middle ear effusion, pleural aspirate, sinus aspirate, sputum and tracheal aspirate. Organisms were identified using conventional methods (optochin susceptibility/bile solubility for S. pneumoniae and X and V factor requirement for H. influenzae). b-Lactamase production was determined for each H. influenzae isolate by a chromogenic cephalosporin (nitrocefin) disc method. Duplicate isolates from the same patient were not accepted.
Isolates were evaluated for antibiotic susceptibility using broth microdilution methodology recommended by CLSI.11
All isolates were tested for susceptibility to amoxicillin/clavulanic acid (2:1; data foramoxicillin alone can be inferred fromdata for S. pneumoniae isolates), azithromycin, cefixime, ceftriaxone, cefuroxime, ciprofloxacin, clarithromycin, levofloxacin and trimethoprim/sulfamethoxazole. S. pneumoniae were additionally tested with penicillin and erythromycin. H. influenzae were also tested with ampicillin. Quality control strains S. pneumoniae ATCC 49619, Escherichia coli ATCC 25922, H. influenzae ATCC 49247, H. influenzae ATCC 49766 and E. coli ATCC 32518 were included on each day of testing. Results of susceptibility testing were accepted if the results of the control strains were within published limits. Susceptibility to the study drugs was calculated based on CLSI breakpoints,12 EUCAST breakpoints13 and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints.14 These breakpoints are shown in Table 1.
Table 1. MIC breakpoints (mg/L) used for S. pneumoniae and H. influenzae isolates
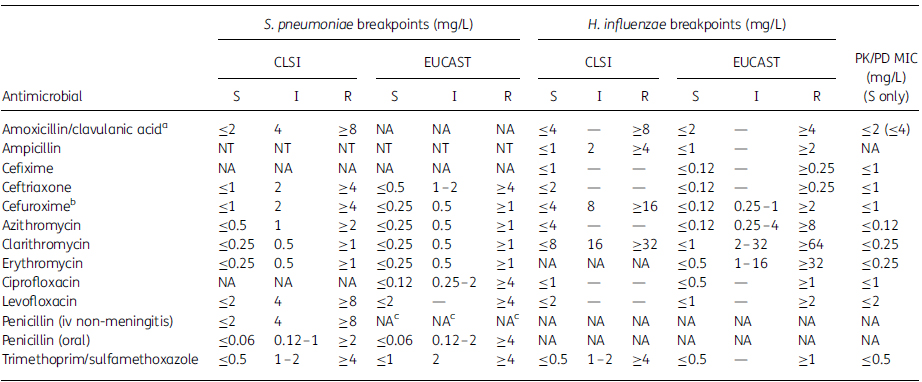
S, susceptible; I, intermediate; R, resistant; NT, not tested; NA, not applicable.
aAmoxicillin/clavulanic acid was tested at a 2:1 amoxicillin to clavulanic acid ratio; breakpoints are expressed as the amoxicillin component. PK/PD breakpoints based on high dose (4 g of amoxicillin with 250 mg of clavulanate per day for adults) shown in parentheses,14 which is the same as CLSI for H. influenzae and one dilution higher for S. pneumoniae.
bBreakpoints used are for cefuroxime axetil.
cEUCAST do not give iv breakpoints but dose-specific susceptible breakpoints are noted for pneumonia: 1.2 g×4 (MIC ≤0.5 mg/L¼susceptible), 1.2 g×6 or 2.4 g×4 (MIC ≤1 mg/L¼susceptible) and 2.4 g×6 (MIC ≤2 mg/L¼susceptible).
Table 2. MIC and percentage susceptibility data for S. pneumoniae isolates (n¼134)
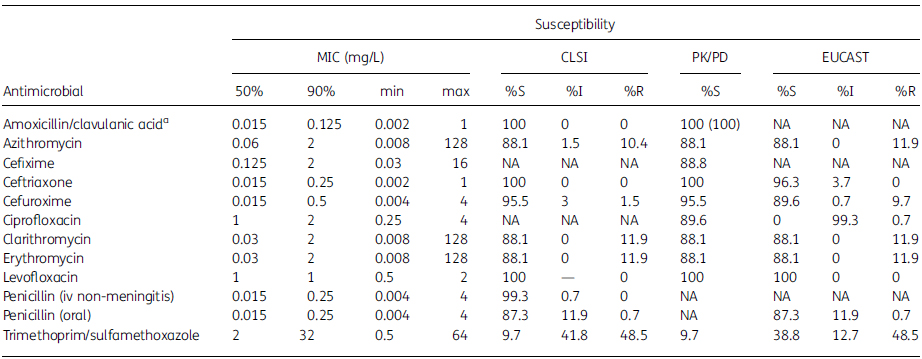
50%, concentration required to inhibit 50% of isolates; 90%, concentration required to inhibit 90% of isolates; min, minimum MIC observed; max, maximum MIC observed; S, susceptible; I, intermediate; R, resistant; NA, not applicable.
aAmoxicillin/clavulanic acid PK/PD susceptibility at high dose shown in parentheses.
Table 3. MIC distribution data for S. pneumoniae isolates (n¼134)
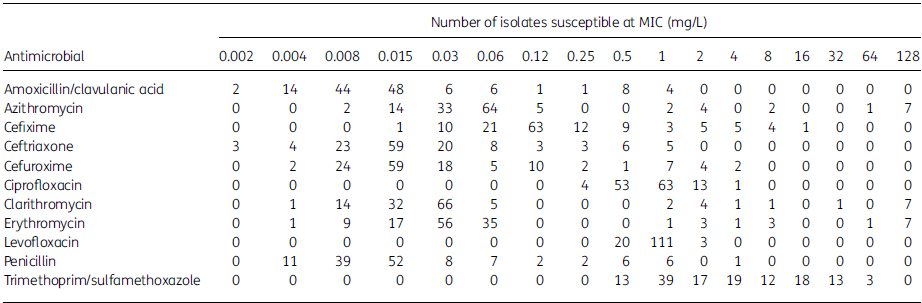
A total of 134 S. pneumoniae isolates were collected fromeight different centres in Ukraine from2011 to 2013. Infection origins of the pneumococci included tracheal aspirates (n¼55; 41.0%), sputum (n¼44; 32.8%) and bronchoalveolar lavage (n¼20; 14.9%). Less frequently, isolates were from sinuses (n¼9; 6.7%), middle ear effusion (n¼4; 3.0%) and pleural aspirates (n¼2; 1.5%). Most isolates (n¼82; 61.2%) came from adult patients (aged 13–64 years), 34 (25.4%) were from children (aged ≤12 years) and 16 (11.9%) were from elderly patients (aged ≥65 years). The remaining two isolates were from patients of unknown age.
Summary MIC and susceptibility data for all 134 S. pneumoniae isolates are shown in Table 2. MIC distribution data are given in Table 3.
Overall, 99.3% (133/134) of S. pneumoniae were penicillin susceptible, 0.7% (1/134) were penicillin intermediate and none was penicillin resistant by CLSI penicillin iv (non-meningitis) breakpoints. However, based on CLSI penicillin oral breakpoints or EUCAST breakpoints 87.3% (117/134) were penicillin susceptible, 11.9% (16/134) were penicillin intermediate and 0.7% (1/134) were penicillin resistant (Table 2). Using EUCAST dose-dependent iv breakpoints, penicillin susceptibility ranged from 94.8% (low dose) to 99.3% (high dose).
All isolates of S. pneumoniae were susceptible to amoxicillin/clavulanic acid (and by inference amoxicillin alone), ceftriaxone and levofloxacin by CLSI and PK/PD breakpoints. Levofloxacin was also 100% active using EUCAST breakpoints but ceftriaxone susceptibility was reduced to 96.3% (129/134) by EUCAST. Cefuroxime susceptibility was slightly lower than ceftriaxone susceptibility, at 95.5% (128/134) by CLSI and PK/PD and 89.6% (120/134) by EUCAST breakpoints. Cefixime breakpoints are not published by CLSI or EUCAST but susceptibility using PK/PD breakpoints was 88.8% (119/134). CLSI guidelines indicate that isolates susceptible to penicillin G (MIC ≤0.06 mg/L) can be reported as susceptible to amoxicillin, amoxicillin/clavulanate, ceftriaxone and cefuroxime. Data from this study confirmed this, as all penicillin-susceptible S. pneumoniae were also susceptible to these b-lactams. However, the reverse was not found. Of the 17 penicillin-non-susceptible isolates, all were susceptible to ceftriaxone and amoxicillin/clavulanic acid (amoxicillin) and 11/17 (64.7%) were susceptible to cefuroxime. A similar 'expert rule' is provided by EUCAST but for penicillins only, i.e. amoxicillin/clavulanate (amoxicillin) in this study. However, unlike CLSI, individual breakpoints are not provided by EUCAST for amoxicillin/clavulanate to make this comparison.
Susceptibility to all three macrolides tested (azithromycin, clarithromycin and erythromycin) was 88.1% (118/134) by CLSI, EUCASTor PK/PD breakpoints. The least active antimicrobial agent tested was trimethoprim/sulfamethoxazole, with only 9.7% of isolates susceptible (13/134) by CLSI or PK/PD breakpoints and 38.8% (52/134) susceptible by EUCAST breakpoints.
Antibiotic susceptibility in S. pneumoniae was also compared according to age group (adult, 13–64 years old; elderly, ≥65 years old; children, ≤12 years old). Susceptibility by age group according to CLSI breakpoints is shown in Figure 1. There was no significant difference for most antibiotics, but trimethoprim/sulfamethoxazole susceptibility was significantly higher in the elderly (37.5%) than in adults (12.2%, P¼0.012) and also significantly higher than in paediatric patients (8.8%, P¼0.014).
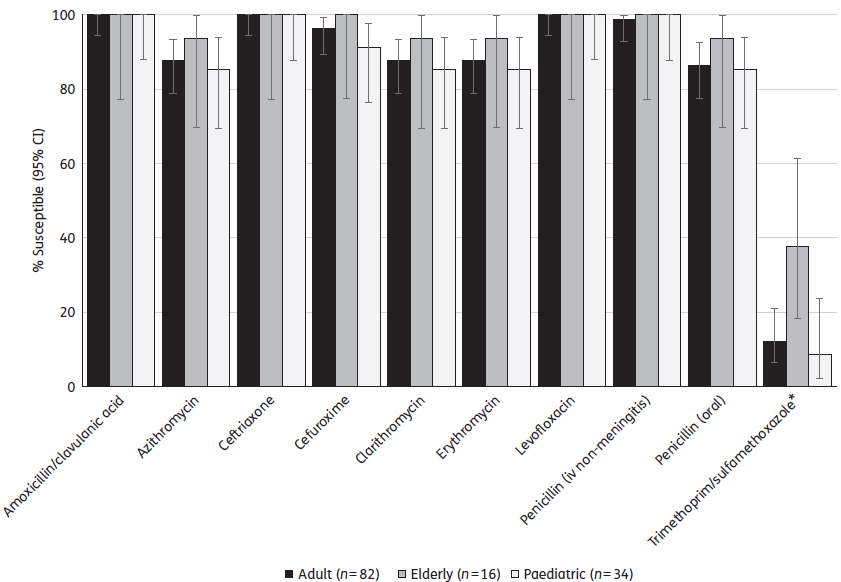
Figure 1. Percentage susceptibility rates (with 95% CIs) for antimicrobials against S. pneumoniae by age group according to CLSI breakpoints. *Trimethoprim/sulfamethoxazole susceptibility was significantly higher in the elderly than in adults (P¼0.012) or paediatric patients (P¼0.014). Age groups: adult, 13–64 years; elderly, ≥65 years; children, ≤12 years.
A total of 67 H. influenzae isolates were collected from eight different centres in Ukraine from 2011–13. Infection origins of the isolates included sputum (n¼56; 83.6%), followed by infrequent isolates from sinuses (n¼7; 10.4%) and bronchoalveolar lavage (n¼4; 6.0%). Most isolates (n¼51; 76.1%) came from adult patients, 8 (11.9%) were from elderly patients and 7 (10.4%) were from children. The remaining isolate was from a patient of unknown age. Three isolates (4.5%) were b-lactamase-positive and none was b-lactamase-negative and ampicillin-resistant (BLNAR).
Summary MIC and susceptibility data for all 67 H. influenzae isolates are shown in Table 4. MIC distribution data are given in Table 5.
Table 4. MIC and percentage susceptibility data for H. influenzae isolates (n¼67)
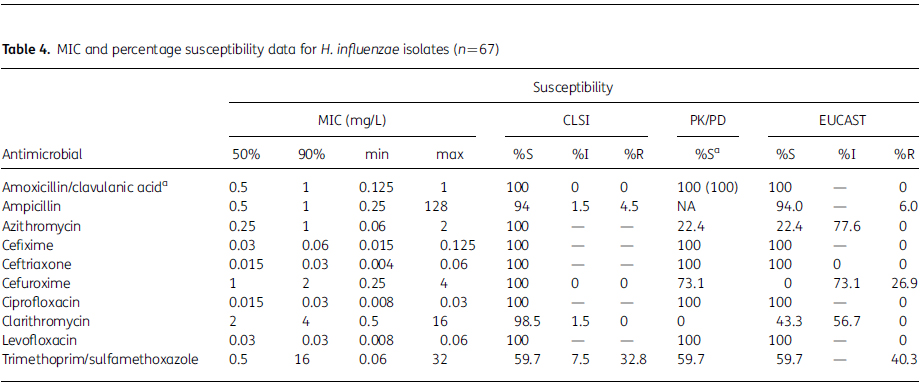
50%, concentration required to inhibit 50% of isolates; 90%, concentration required to inhibit 90% of isolates; min, minimum MIC observed; max, maximum MIC observed; S, susceptible; I, intermediate; R, resistant; NA, not applicable.
aAmoxicillin/clavulanic acid PK/PD susceptibility at high dose shown in parentheses.
Table 5. MIC distribution data for all H. influenzae isolates (n¼67)
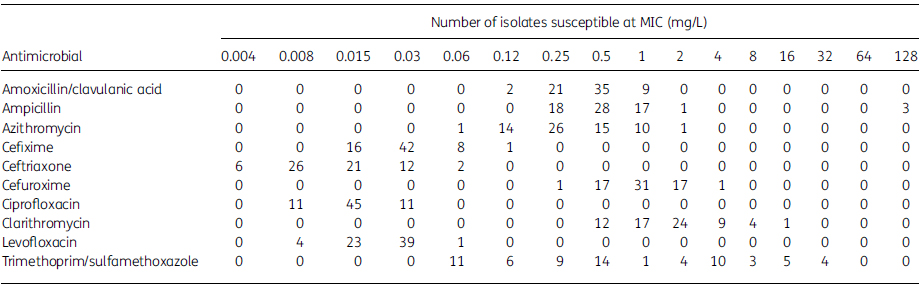
Susceptibility was 100% for amoxicillin/clavulanic acid, ceftriaxone, ciprofloxacin and levofloxacin according to all three breakpoint guidelines. Cefuroxime susceptibility was also 100% by CLSI, but this reduced to 73.1% (49/67) by EUCAST and PK/PD. For the macrolides a similarly high susceptibility was observed by CLSI: azithromycin (100%) and clarithromycin (98.5%, 66/67). This was dramatically reduced by PK/PD breakpoints: azithromycin (22.4% susceptible, 15/67) and clarithromycin (0% susceptible) and EUCAST breakpoints: azithromycin (22.4% susceptible, 15/67) and clarithromycin (43.3% susceptible, 29/67). The least active agent was trimethoprim/sulfamethoxazole, at 59.7% susceptible (40/67) by all three breakpoint categories.
The increasing prevalence of antimicrobial resistance among the major community respiratory tract pathogens is a serious global problem that complicates the management of these infections. SOAR surveys antimicrobial resistance in key respiratory pathogens and is currently conducted throughout the Middle East, Africa, Latin America, Asia-Pacific and the Commonwealth of Independent States countries and has been running since 2002. It was established to provide information on local resistance patterns among the two most common pulmonary pathogens, S. pneumoniae and H. influenzae.
S. pneumoniae resistance to antibiotics is a growing problem and has become an important prognostic factor since it is directly associated with persistent disease or disease mortality.15,16 In this study, with lower oral dosing, 87.3% of pneumococcal isolates were found to be susceptible to penicillin using CLSI oral breakpoints. Therefore, higher iv dosing is required to get susceptibility close to 100%. However, for many patients with community-acquired respiratory tract infections an oral therapy is desired. For this option amoxicillin/clavulanic acid (or amoxicillin alone, which would be expected to have identical activity) and levofloxacin remain the only orally available agents with 100% susceptibility. However, fluoroquinolones are not recommended for the treatment of paediatric patients. Cefuroxime is also orally bioavailable but susceptibility was lower, at 95.5% (CLSI or PK/PD) and 89.6% (EUCAST). The data from this study confirm that isolates of S. pneumoniae susceptible to penicillin G are also susceptible to other penicillins as inferred by CLSI and EUCAST guidelines and cephalosporins as inferred by CLSI guidelines. Although penicillin resistance was relatively low in Ukraine, the data from this study showed the reverse was not correct using CLSI breakpoints; i.e. penicillin-non-susceptible S. pneumoniae were susceptible to other b-lactams—the most significant being ceftriaxone. Therefore, either the b-lactam breakpoints are not correct or the CLSI cross-resistance statement within the b-lactam class is not correct. This has also been found in other countries with higher penicillin resistance (SOAR 2011–13 data in other countries, as seen in other articles in this Supplement) and warrants further investigation. The pneumococci were universally 88.1% susceptible to the macrolides azithromycin, clarithromycin and erythromycin and therefore, although these agents can be given orally, they do not provide the same level of coverage as that observed with amoxicillin/clavulanic acid (amoxicillin) or levofloxacin. The least active antimicrobial tested was trimethoprim/sulfamethoxazole, where susceptibility was ,10% (CLSI or PK/PD), but this was 38.8% by EUCAST due to a one dilution higher breakpoint. Therefore trimethoprim/sulfamethoxazole can be considered inactive against pneumococci. In addition, this was the only antimicrobial agent to have significantly different susceptibility by age group, although the most susceptible age group (elderly) was still only 37.5% susceptible.
H. influenzae from Ukraine were generally highly susceptible to most antimicrobial agents, including ampicillin, due to a low b-lactamase prevalence (6%). As with S. pneumoniae, trimethoprim/sulfamethoxazole activity was weak. Following CLSI recommendations, susceptibility to the macrolides was 98.5%–100%. However, analysis by PK/PD breakpoints indicates zero susceptibility to clarithromycin and only 22.4% susceptibility to azithromycin. EUCAST breakpoints for azithromycin also showed 22.4% susceptibility and a slightly higher susceptibility to clarithromycin (43.3%). These data illustrate the continued need for the harmonization of clinical breakpoints, especially in countries where clinical laboratories may follow CLSI or EUCAST guidelines.
There are few other publications on antimicrobial resistance in respiratory pathogens from Ukraine, but the data from this study suggest that resistance levels are low in Ukraine compared with other parts of Eastern Europe. For example, the penicillin nonsusceptibility rate in S. pneumoniae from Romania in 2008 was 50% and macrolide resistance was 30.9%.17 Even as far back as 2001, resistance in respiratory pathogens from Romania was reported to be higher than that currently seen in Ukraine.18 Similarly, ampicillin non-susceptibility in respiratory isolates of H. influenzae from Bulgaria is cited at 18%.19 Future studies are warranted to confirm continued low resistance levels in these respiratory pathogens from Ukraine.
The main limitations of this study stem from small sample sizes and the attempt to draw conclusions about antimicrobial susceptibility levels in the country using data from two hospital sites. However, despite these limitations, patterns emerged that can be useful for clinicians in selecting empirical therapy for community-acquired respiratory tract infections.
We would like to thank the participating laboratories for their susceptibility data used in this analysis (Yuriy Mostovoy, Mykola Ostrovsky, Andriy Vertegel, Anatoliy Kosakovsky, Maryna Zakharova, Fedir Yurochko, Nadiya Rudnitska) and Dr Keith Barker (GSK) for reviewing the manuscript.
The data were presented as a poster abstract during the 24th ECCMID Congress in Barcelona, Spain (Abstract number P-1589).
This study was funded by GlaxoSmithKline.
This article forms part of a Supplement sponsored by GlaxoSmithKline.
D. Torumkuney and G. Gladka are employees of GlaxoSmithKline. D. Torumkuney also holds shares in GlaxoSmithKline. I. Morrissey is an employee of IHMA, a medical communication and consultancy company, who participated in the exploration, interpretation of the results and preparation of thismanuscript on behalf of GSK. IHMA also provided medical writing support in the form of writing assistance, collating author's comments, grammatical editing and referencing that was paid for by GSK. All other authors declare that they have no conflict of interest.
Editorial assistance was provided by Tracey Morris, Livewire Editorial Communications.
SHDM.FORUM'24: Головна подія року!
Конференція MEDONNA
Хронічні запальні захворювання ЛОР органів і біоплівки. Взаємозв'язок і можливості впливу
Переглянувши відео, ви дізнаєтеся про те, чим відрізняється оригінальний препарат від генерика, про безпечність та ефективність даних ліків. Також подана інформація про ізомери, допоміжні речовини, технологію виробництва, біодоступність і біоеквівалентність оригінальних ліків та генериків.
Інформація, опублікована на даному сайті, орієнтована на загальне ознайомлення та жодним чином не може бути використана в якості медичних, практичних або комерційних рекомендацій. У зв’язку з цим, Сайт «Школи доказової медицини» не несе жодної відповідальності за негативні наслідки, отримані через використання матеріалів, викладених на даному сайті. Документація з фармацевтичних продуктів не є рекламою та не призначена для того, щоб використовувати її замість консультації з кваліфікованими фахівцями в галузі медицини та інших галузях. Документація з фармацевтичних продуктів надається за вашою згодою відповідно до вимог ч.ч. 1, 2 ст. 15 Закону України «Про захист прав споживачів» від 12.05.1991 р. № 1023-XII. Якщо вам потрібна консультація з конкретного питання, пов’язаного зі здоров’ям, необхідно звернутися до фахівців- професіоналів.
Продовжуючи своє перебування на сайті, ви підтверджуєте свою згоду на дистанційне отримання інформації про лікарські та косметичні засоби (включаючи інформацію про рецептурні лікарські засоби) на підставі вимог ч.ч. 1, 2 ст. 15 Закону України «Про захист прав споживачів» від 12.05.1991 р. № 1023-XII.
Уся інформація, яка міститься на даному сайті, подана з освітньою метою виключно для медичних та фармацевтичних працівників і не замінює консультації лікаря.
