Management, reasons for failure of medical and surgical therapy
in Chronic Rhinosinusitis
In this chapter a differentiation is made between CRSsNP and
CRSwNP. Readers have to realize that often in studies no clear
difference is made between these two patients groups. Sometimes
for this reason studies are discussed in both the parts on
CRSsNP as the parts of CRSwNP.
6.1. Treatment of CRSsNP with corticosteroids
6.1.1. Introduction
The introduction of topically administered glucocorticoids has improved the treatment of upper (rhinitis, nasal polyps) and lower (asthma) airway inflammatory disease. The clinical efficacy of glucocorticoids may depend in part on their ability to reduce airway eosinophil infiltration by preventing their increased viability and activation. Both topical and systemic glucocorticoids may affect the eosinophil function by both directly reducing eosinophil viability and activation (899, 1643-1645) or indirectly reducing the secretion of chemotactic cytokines by nasal mucosa and polyp epithelial cells (1646-1649). The biological action of glucocorticoids is mediated through activation of intracellular glucocorticoid receptors (GR) (1650, 1651) expressed in many tissues and cells (1652). Two human isoforms of GR have been identified, GRα and GRβ, which originate from the same gene by alternative splicing of the GR primary transcript (1653). Upon hormone binding, GRα enhances anti-inflammatory or represses pro-inflammatory gene transcription, and exerts most of the anti-inflammatory effects of glucocorticoids through protein-protein interactions between GR and transcription factors, such as AP-1 and NF-κB. The GRβ isoform does not bind steroids but may interfere with the GR function. There may be several mechanisms accounting for the resistance to the anti-inflammatory effects of glucocorticoids, including an overexpression of GRβ or a down-expression of GRα. Increased expression of GRβ has been reported in patients with nasal polyps (1654, 1655) while down-regulation of GR levels after treatment with glucocorticoids (1656, 1657) has also been postulated to be one of the possible explanations for the secondary glucocorticoid resistance phenomenon.
The ability of the drug to reach the appropriate anatomic region on the para-nasal system has been the subject of much research in the past 5 years. While systemic delivery is available, effective topical therapy relies on several factors. Delivery technique, surgical state of the sinus cavity, delivery device and fluid dynamics (volume, pressure, position) have a significant impact on the delivery of topical therapies to the sinus mucosa. Distribution of topical solution to the unoperated sinuses is limited (1658) and in the setting of CRS with mucosal oedema it is probably only in the order of <2% of total irrigation volume (1659). Nebulization is also ineffective with <3% sinus penetration (1660). A fundamentally held belief amongst those treating CRS patients is that Endoscopic sinus surgery (ESS) improves the delivery of topical medications to the sino-nasal mucosa (1661, 1662), yet only recent evidence exists to support this claim (1658, 1663). Endoscopic sinus surgery is essential to effectively allow topical distribution to the sinuses. The frontal and sphenoid sinus are essentially inaccessible prior to surgery (1658) and an ostial size of 4mm+ is required to even begin penetration to the maxillary sinus (1658). For delivery, nebulizers poorly penetrate the sinuses even after maximal ESS (1664) and large volume squeeze bottles or passive flow devices appear to have the best efficacy post ESS (1658, 1661, 1662, 1664). Pre-surgery, the distribution to the sinuses is extremely limited regardless of device (1658, 1659, 1663) and sprays are the least effective of all (1658). Post-surgery distribution is superior with high volume positive pressure devices (1658, 1659, 1663). Simple low volume sprays and drops have very poor distribution and should be considered a nasal cavity treatment only, especially prior to ESS (1658). Although multiple devices and head positions have been trialled, less than 50% of most low volume applications will reach even the middle meatus (1665). There is limited data on the exact volume required to allow complete distribution. Higher volumes do appear to penetrate both maxillary and frontal sinus with good coverage starting at about 100ml (1666). The frontal and sphenoid sinuses are not accessed well by pressurized spray when compared to high volume devices such as squeeze bottles or neti pots (1658). Higher volume and positive pressure irrigation is likely to result in the best distribution from current research.
The anti-inflammatory effect of corticosteroids could, theoretically, be expected to benefit all forms of rhinosinusitis. Considering the abundance of publications on the use of corticosteroids in CRSsNP and CRSwNP, we present the findings from level 1 studies. Where no level 1 study exists, a summary of available evidence is presented. Data is presented separately on CRSsNP and CRSwNP along with local and systemic use.
6.1.2. Local corticosteroid (INCS) in CRSsNP
The use of local intranasal corticosteroid (INCS) has been widely published for many years and the following summary is based on a systematic search and summary of level 1 or randomized controlled trials for the evidence of benefit for symptoms in treating CRSsNP with INCS. However, not all studies demonstrate a benefit and a subgroup analysis is performed to help elucidate the reasons for some authors findings benefit over others.
6.1.2.1. Inclusion criteria and exclusion criteria Local corticosteroid (INCS) in CRSsNP
Inclusion criteria
Participants in the trials have to be defined as having chronic rhinosinusitis (CRS) by either:
- Trials which included participants with CRS both with and without polyps if the majority of participants were without polyps. If possible, we only extracted data for participants with CRS without polyps.
Exclusion criteria
6.1.2.2. Types of interventions Local corticosteroid (INCS) in CRSsNP
6.1. Treatment of CRSsNP with corticosteroids
6.1.1. Introduction
The introduction of topically administered glucocorticoids has improved the treatment of upper (rhinitis, nasal polyps) and lower (asthma) airway inflammatory disease. The clinical efficacy of glucocorticoids may depend in part on their ability to reduce airway eosinophil infiltration by preventing their increased viability and activation. Both topical and systemic glucocorticoids may affect the eosinophil function by both directly reducing eosinophil viability and activation (899, 1643-1645) or indirectly reducing the secretion of chemotactic cytokines by nasal mucosa and polyp epithelial cells (1646-1649). The biological action of glucocorticoids is mediated through activation of intracellular glucocorticoid receptors (GR) (1650, 1651) expressed in many tissues and cells (1652). Two human isoforms of GR have been identified, GRα and GRβ, which originate from the same gene by alternative splicing of the GR primary transcript (1653). Upon hormone binding, GRα enhances anti-inflammatory or represses pro-inflammatory gene transcription, and exerts most of the anti-inflammatory effects of glucocorticoids through protein-protein interactions between GR and transcription factors, such as AP-1 and NF-κB. The GRβ isoform does not bind steroids but may interfere with the GR function. There may be several mechanisms accounting for the resistance to the anti-inflammatory effects of glucocorticoids, including an overexpression of GRβ or a down-expression of GRα. Increased expression of GRβ has been reported in patients with nasal polyps (1654, 1655) while down-regulation of GR levels after treatment with glucocorticoids (1656, 1657) has also been postulated to be one of the possible explanations for the secondary glucocorticoid resistance phenomenon.
The ability of the drug to reach the appropriate anatomic region on the para-nasal system has been the subject of much research in the past 5 years. While systemic delivery is available, effective topical therapy relies on several factors. Delivery technique, surgical state of the sinus cavity, delivery device and fluid dynamics (volume, pressure, position) have a significant impact on the delivery of topical therapies to the sinus mucosa. Distribution of topical solution to the unoperated sinuses is limited (1658) and in the setting of CRS with mucosal oedema it is probably only in the order of <2% of total irrigation volume (1659). Nebulization is also ineffective with <3% sinus penetration (1660). A fundamentally held belief amongst those treating CRS patients is that Endoscopic sinus surgery (ESS) improves the delivery of topical medications to the sino-nasal mucosa (1661, 1662), yet only recent evidence exists to support this claim (1658, 1663). Endoscopic sinus surgery is essential to effectively allow topical distribution to the sinuses. The frontal and sphenoid sinus are essentially inaccessible prior to surgery (1658) and an ostial size of 4mm+ is required to even begin penetration to the maxillary sinus (1658). For delivery, nebulizers poorly penetrate the sinuses even after maximal ESS (1664) and large volume squeeze bottles or passive flow devices appear to have the best efficacy post ESS (1658, 1661, 1662, 1664). Pre-surgery, the distribution to the sinuses is extremely limited regardless of device (1658, 1659, 1663) and sprays are the least effective of all (1658). Post-surgery distribution is superior with high volume positive pressure devices (1658, 1659, 1663). Simple low volume sprays and drops have very poor distribution and should be considered a nasal cavity treatment only, especially prior to ESS (1658). Although multiple devices and head positions have been trialled, less than 50% of most low volume applications will reach even the middle meatus (1665). There is limited data on the exact volume required to allow complete distribution. Higher volumes do appear to penetrate both maxillary and frontal sinus with good coverage starting at about 100ml (1666). The frontal and sphenoid sinuses are not accessed well by pressurized spray when compared to high volume devices such as squeeze bottles or neti pots (1658). Higher volume and positive pressure irrigation is likely to result in the best distribution from current research.
The anti-inflammatory effect of corticosteroids could, theoretically, be expected to benefit all forms of rhinosinusitis. Considering the abundance of publications on the use of corticosteroids in CRSsNP and CRSwNP, we present the findings from level 1 studies. Where no level 1 study exists, a summary of available evidence is presented. Data is presented separately on CRSsNP and CRSwNP along with local and systemic use.
6.1.2. Local corticosteroid (INCS) in CRSsNP
The use of local intranasal corticosteroid (INCS) has been widely published for many years and the following summary is based on a systematic search and summary of level 1 or randomized controlled trials for the evidence of benefit for symptoms in treating CRSsNP with INCS. However, not all studies demonstrate a benefit and a subgroup analysis is performed to help elucidate the reasons for some authors findings benefit over others.
6.1.2.1. Inclusion criteria and exclusion criteria Local corticosteroid (INCS) in CRSsNP
Inclusion criteria
Participants in the trials have to be defined as having chronic rhinosinusitis (CRS) by either:
- European Position Paper on Rhinosinusitis and Nasal Polyps 2007 (8);
- or Rhinosinusitis Task Force Report (523) and its revision (1667);
- or having chronic sino-nasal symptoms for longer than 12 weeks.
- Trials which included participants with CRS both with and without polyps if the majority of participants were without polyps. If possible, we only extracted data for participants with CRS without polyps.
Exclusion criteria
- Patients defined by the study authors as having acute or recurrent-acute sinusitis.
- Patients defined by the study authors as having CRS with polyps or nasal polyposis.
- Patients had CRS both with and without polyps and the majority of participants had polyps
6.1.2.2. Types of interventions Local corticosteroid (INCS) in CRSsNP
- Any dose of topical steroid versus placebo.
- Any dose of topical steroid versus no treatment.
- Any dose of topical steroid versus alternative topical steroid.
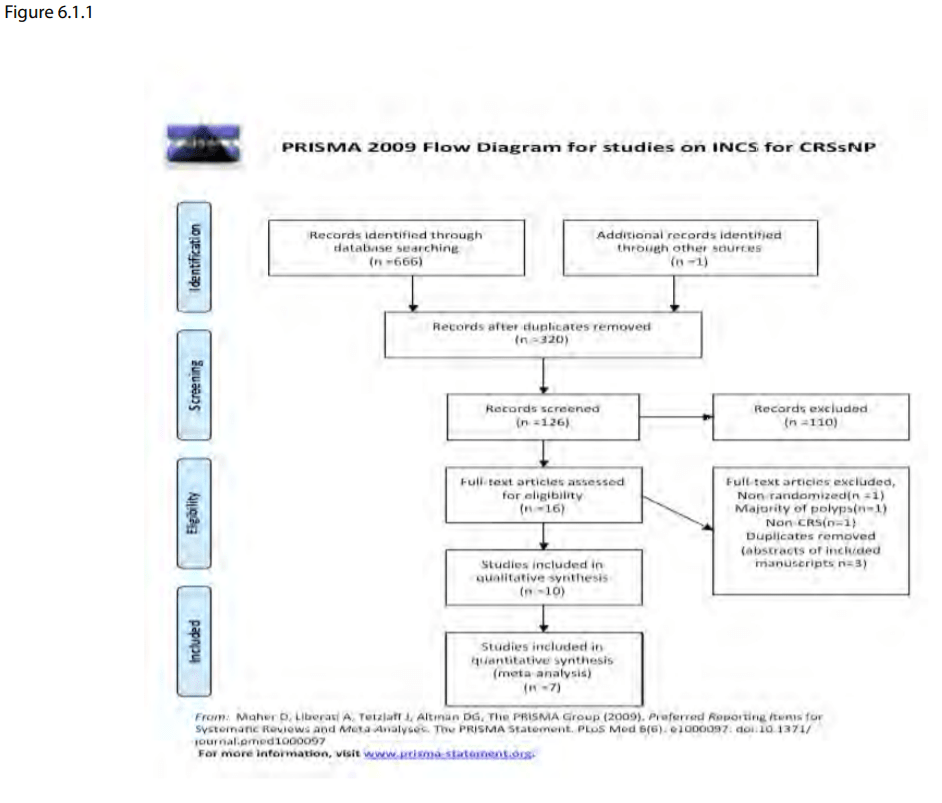
6.1.2.3. Flow chart
A total of 666 references from the searches: 541 of these were removed in first-level screening (i.e. removal of duplicates and clearly irrelevant references), leaving 125 references for further consideration. We subsequently found one additional trial from a manual search guided by the identified references. A flow chart of study retrieval and selection is provided as Figure 6.1.1.
A total of 666 references from the searches: 541 of these were removed in first-level screening (i.e. removal of duplicates and clearly irrelevant references), leaving 125 references for further consideration. We subsequently found one additional trial from a manual search guided by the identified references. A flow chart of study retrieval and selection is provided as Figure 6.1.1.
6.1.2.5. Included studies
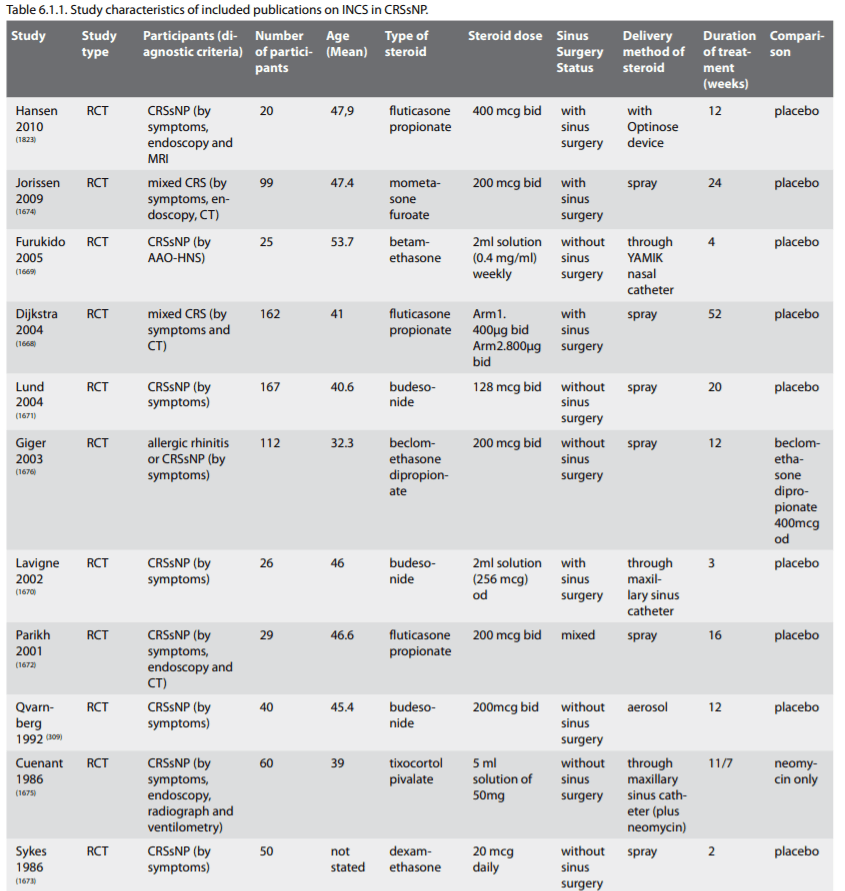
Ten studies with a total of 590 patients met the inclusion criteria. The characteristics of included studies are listed as Table 6.1.1.
Ten studies with a total of 590 patients met the inclusion criteria. The characteristics of included studies are listed as Table 6.1.1.
6.1.2.6. Summary of data
There were 11 included studies. Nine trials (80%) compared topical steroid against placebo (Hansen 2010; Dijkstra 2004; Furukido 2005; Jorissen 2009; Lavigne 2002; Lund 2004; Parikh 2001; Qvarnberg 1992; Sykes 1986) (309, 1668-1674, 1823). One trial (10%) (1675) with 112 patients compared two treatment regimes of steroid administration without comparing to placebo. One (10%) trial (1676) with 60 patients compared topical steroid with antibiotic against antibiotic alone. We found no trials comparing topical steroid versus alternative topical steroid.
Five included studies were sponsored by pharmaceutical companies. Two were fully and three were partly supported as follows: Dijkstra 2004 (1668) (GlaxoSmithKline (GSK), Jorrisen 2009 (1674) (Schering-Plough Corp), Hansen 2010 (1823) (Optinose UK ltd), Lund 2004 (1671) (AstraZeneca and R&D Lund) and Lavigne 2002 (1670) (AstraZeneca Canada Inc and Fon de Recherche en Sante du Quebec). Medications were supplied by pharmaceutical companies in three studies: Parikh 2001 (1672) (Glaxo Wellcome Research), Sykes 1986 (1673) (Boehringer Ingelheim), Qvarnberg 1992 (309) (Suomen Astra OY). Furukido 2005 (1669) was not funded by pharmaceutical companies. Two studies did not state how they were funded (Cuenant 1986; Giger 2003) (1675, 1676). A summary of outcomes is provided in Table 6.1.2. with the majority demonstrating a benefit to the use of INCS.
6.1.3.1. Meta-analysis
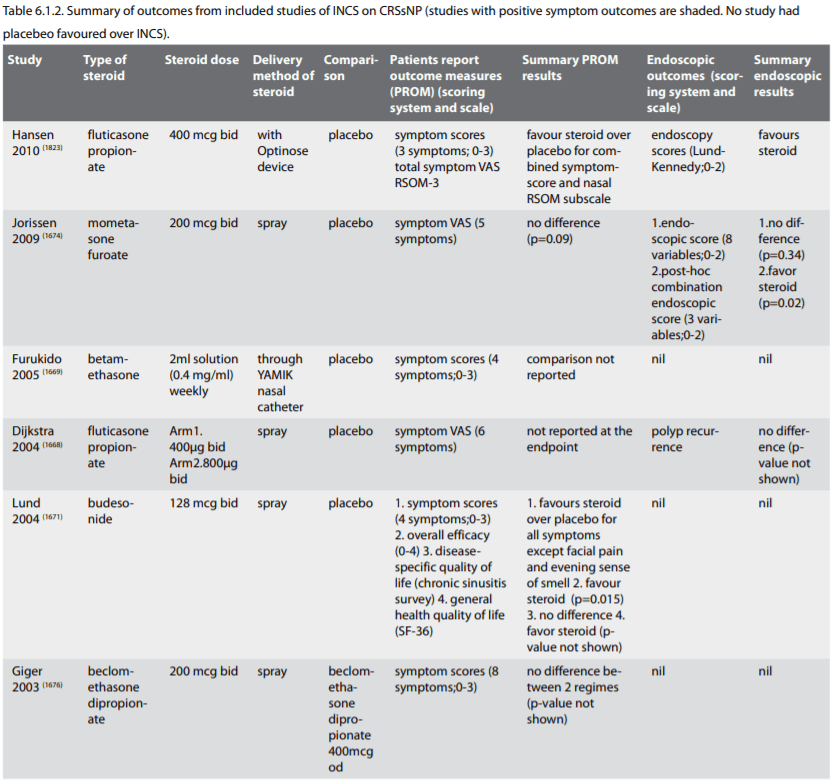
Of the eight studies comparing INCS to placebo, Five studies (Furukido 2005; Jorissen 2009; Lavigne 2002; Lund 2004; Parikh 2001); (1669-1672, 1674) and could be combined in the meta-analysis. Pooled data analyses of symptom scores and proportion of responding patients demonstrated significant benefit in the topical steroid group. The pooled results significantly favoured the topical steroid group (combined standardised mean difference (SMD -0.37; 95% confidence interval (CI) -0.60 to -0.13, p=0.002; five trials, 286 patients) The I2 was 12%, suggesting no heterogeneity (x2 = 4.57, degrees of freedom (df) = 4, p=0.33). This was true for both SMD and responder analysis (Figure 6.1.2a & 6.1.2b). The four studies that did not provide data for meta-analysis were (309, 1673, 1677, 1823) and only Dijkstra 2004 did not favour INCS.
Endoscopic scores were report in only 2 studies (Jorissen 2009 and Parikh 2001) (1672, 1674) and did not reach significant outcome on meta-analysis. Three studies used non-validated radiologic outcomes (Furukido 2005, Qvarnberg 1992, Sykes 1986) (309, 1669, 1673) and these all had no benefit favouring INCS but could not be combined for meta-analysis.
The standardised mean difference (SMD) and 95% CIs for continuous data such as post-intervention scores or change in symptom scores. The risk ratio (RR) and 95% CI of responsiveness was used at a specific time point for dichotomous data such as number of patients responding to treatment or number of patients having positive radiographs. The intervention effects were pooled when trials were sufficiently homogeneous. A fixed-effect model was used and assumed that each study was estimating the same quantity.
There were 11 included studies. Nine trials (80%) compared topical steroid against placebo (Hansen 2010; Dijkstra 2004; Furukido 2005; Jorissen 2009; Lavigne 2002; Lund 2004; Parikh 2001; Qvarnberg 1992; Sykes 1986) (309, 1668-1674, 1823). One trial (10%) (1675) with 112 patients compared two treatment regimes of steroid administration without comparing to placebo. One (10%) trial (1676) with 60 patients compared topical steroid with antibiotic against antibiotic alone. We found no trials comparing topical steroid versus alternative topical steroid.
Five included studies were sponsored by pharmaceutical companies. Two were fully and three were partly supported as follows: Dijkstra 2004 (1668) (GlaxoSmithKline (GSK), Jorrisen 2009 (1674) (Schering-Plough Corp), Hansen 2010 (1823) (Optinose UK ltd), Lund 2004 (1671) (AstraZeneca and R&D Lund) and Lavigne 2002 (1670) (AstraZeneca Canada Inc and Fon de Recherche en Sante du Quebec). Medications were supplied by pharmaceutical companies in three studies: Parikh 2001 (1672) (Glaxo Wellcome Research), Sykes 1986 (1673) (Boehringer Ingelheim), Qvarnberg 1992 (309) (Suomen Astra OY). Furukido 2005 (1669) was not funded by pharmaceutical companies. Two studies did not state how they were funded (Cuenant 1986; Giger 2003) (1675, 1676). A summary of outcomes is provided in Table 6.1.2. with the majority demonstrating a benefit to the use of INCS.
6.1.3.1. Meta-analysis
Of the eight studies comparing INCS to placebo, Five studies (Furukido 2005; Jorissen 2009; Lavigne 2002; Lund 2004; Parikh 2001); (1669-1672, 1674) and could be combined in the meta-analysis. Pooled data analyses of symptom scores and proportion of responding patients demonstrated significant benefit in the topical steroid group. The pooled results significantly favoured the topical steroid group (combined standardised mean difference (SMD -0.37; 95% confidence interval (CI) -0.60 to -0.13, p=0.002; five trials, 286 patients) The I2 was 12%, suggesting no heterogeneity (x2 = 4.57, degrees of freedom (df) = 4, p=0.33). This was true for both SMD and responder analysis (Figure 6.1.2a & 6.1.2b). The four studies that did not provide data for meta-analysis were (309, 1673, 1677, 1823) and only Dijkstra 2004 did not favour INCS.
Endoscopic scores were report in only 2 studies (Jorissen 2009 and Parikh 2001) (1672, 1674) and did not reach significant outcome on meta-analysis. Three studies used non-validated radiologic outcomes (Furukido 2005, Qvarnberg 1992, Sykes 1986) (309, 1669, 1673) and these all had no benefit favouring INCS but could not be combined for meta-analysis.
The standardised mean difference (SMD) and 95% CIs for continuous data such as post-intervention scores or change in symptom scores. The risk ratio (RR) and 95% CI of responsiveness was used at a specific time point for dichotomous data such as number of patients responding to treatment or number of patients having positive radiographs. The intervention effects were pooled when trials were sufficiently homogeneous. A fixed-effect model was used and assumed that each study was estimating the same quantity.

6.1.3.2. Subgroup analysis
Subgroup analysis was performed as follows.
• Topical delivery method
- Nasal (drops, sprays, nebulisers) versus sinus (direct cannulation, irrigation post-surgery) delivery method.
Low volume (defined as any simple spray volume approximating < 1 ml) versus large volume (defined as any significant volume > 60 ml - representing a simple irrigation syringe or smallest commercial irrigation device. We pre-defined low and large volume based on previous studies showing how the volume applied affects sinus delivery (1666). Low pressure (including spray, nebulisers, instilled solution through a tube and non-pressure irrigation) versus high pressure (including positive pressure irrigation).
Differences between the two subgroups for fixed-effect analyses were based on the inverse-variance method in the case of continuous data and the Mantel-Haenszel method in the case of dichotomous data.
There was a benefit on subgroup analysis for INCS delivery method. This was significant when sinus delivery methods (SMD -1.32; 95% CI -2.26 to -0.38) were compared to nasal delivery methods (SMD -0.30; 95% -0.55 to -0.06) (p=0.04). Similar findings were seen in responders as well as SMD analysis (Figure 6.1.3.a and 6.1.3.b). There were no studies using nasal drops and thus no comparison is made. No high volume and high pressure topical delivery techniques (i.e. irrigation or atomizer) were described.
Subgroup analysis was performed as follows.
• Topical delivery method
- Nasal (drops, sprays, nebulisers) versus sinus (direct cannulation, irrigation post-surgery) delivery method.
Low volume (defined as any simple spray volume approximating < 1 ml) versus large volume (defined as any significant volume > 60 ml - representing a simple irrigation syringe or smallest commercial irrigation device. We pre-defined low and large volume based on previous studies showing how the volume applied affects sinus delivery (1666). Low pressure (including spray, nebulisers, instilled solution through a tube and non-pressure irrigation) versus high pressure (including positive pressure irrigation).
- Surgical status
- Corticosteroid type
Differences between the two subgroups for fixed-effect analyses were based on the inverse-variance method in the case of continuous data and the Mantel-Haenszel method in the case of dichotomous data.
There was a benefit on subgroup analysis for INCS delivery method. This was significant when sinus delivery methods (SMD -1.32; 95% CI -2.26 to -0.38) were compared to nasal delivery methods (SMD -0.30; 95% -0.55 to -0.06) (p=0.04). Similar findings were seen in responders as well as SMD analysis (Figure 6.1.3.a and 6.1.3.b). There were no studies using nasal drops and thus no comparison is made. No high volume and high pressure topical delivery techniques (i.e. irrigation or atomizer) were described.
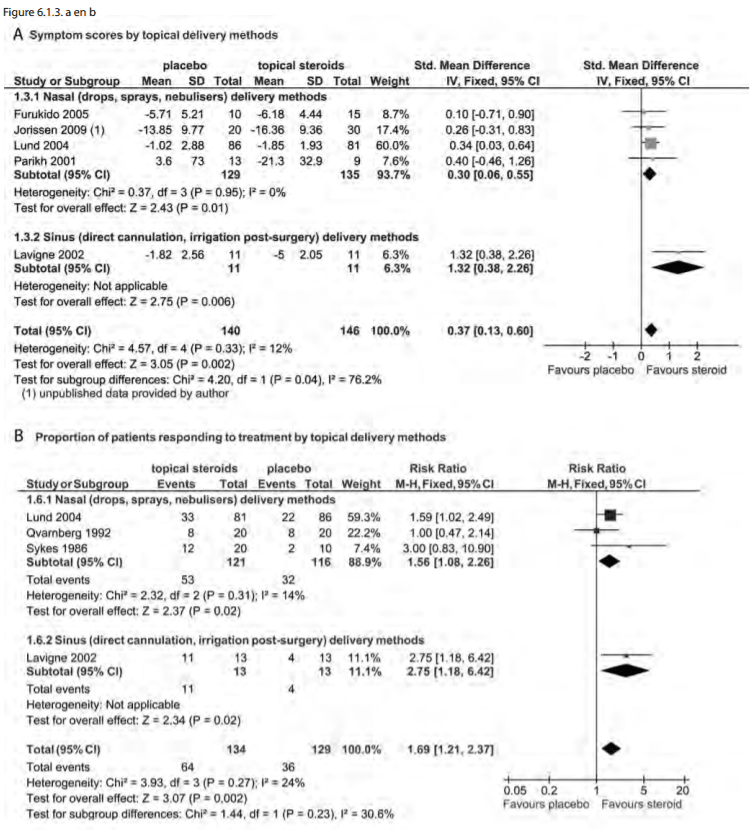
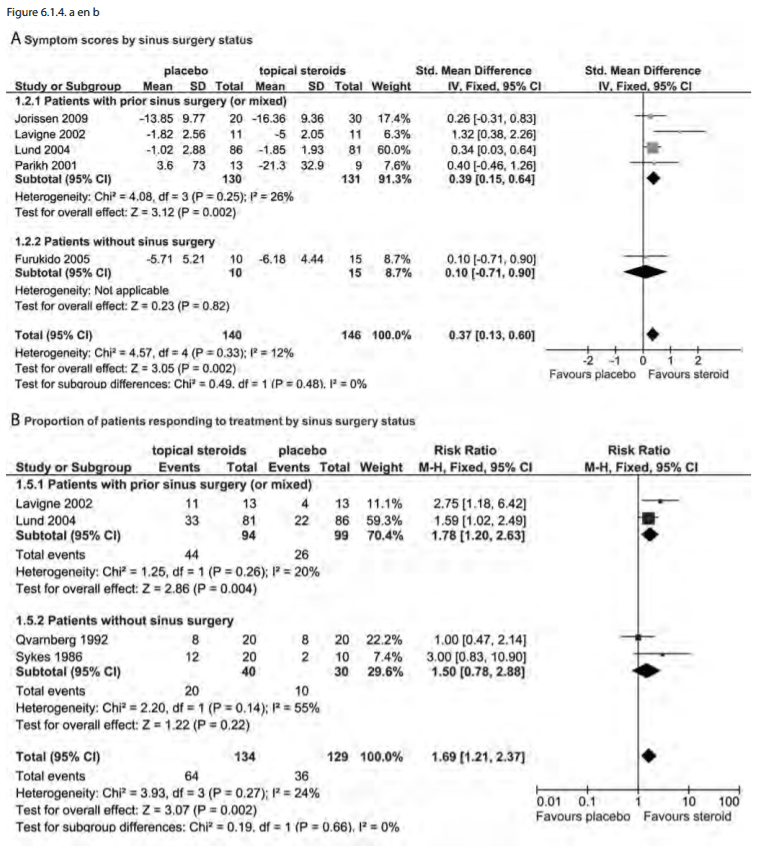
When the surgical state of the patients was assessed on
subgroup, only patients with prior surgery for CRSsNP had
symptom improvement (SMD-0.54 CI -1.03, -0.06)) but there was no
improvement for those patients without surgery (SMD -0.10,
-0.90, 0.71). The comparative assessment between subgroups did
not reach significance (p=0.23). This was true for responders as
well as SMD (Figures 6.1.4.a and 6.1.4.b).
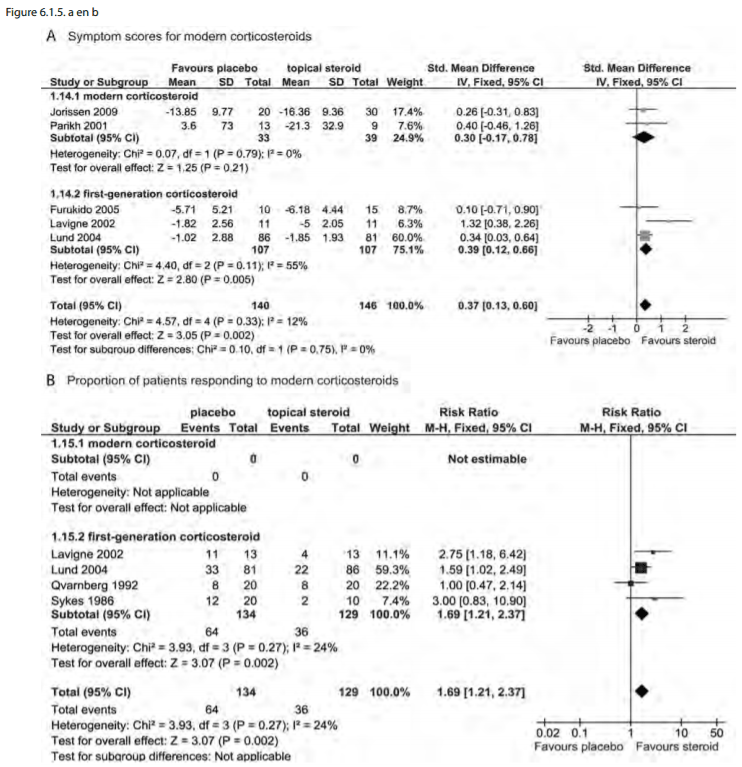
Finally, by corticosteroid type, there were 3 studies using
modern corticosteroids (1674, 1668,1672) compared to 7 with
older first-generation corticosteroid types. Only symptom scores
were available for comparison with no significant difference
between subgroups (p=0.75). Although, it may appear that the
early generation INCS perform better than modern on the forest
plot (Figure 6.1.5.a and 6.1.5.b) this difference is not
significant and there are no data from modern INCS to use in the
proportion of responders analysis.
6.1.4. Side-effects of local corticosteroid chronic
rhinosinusitis without nasal polyps
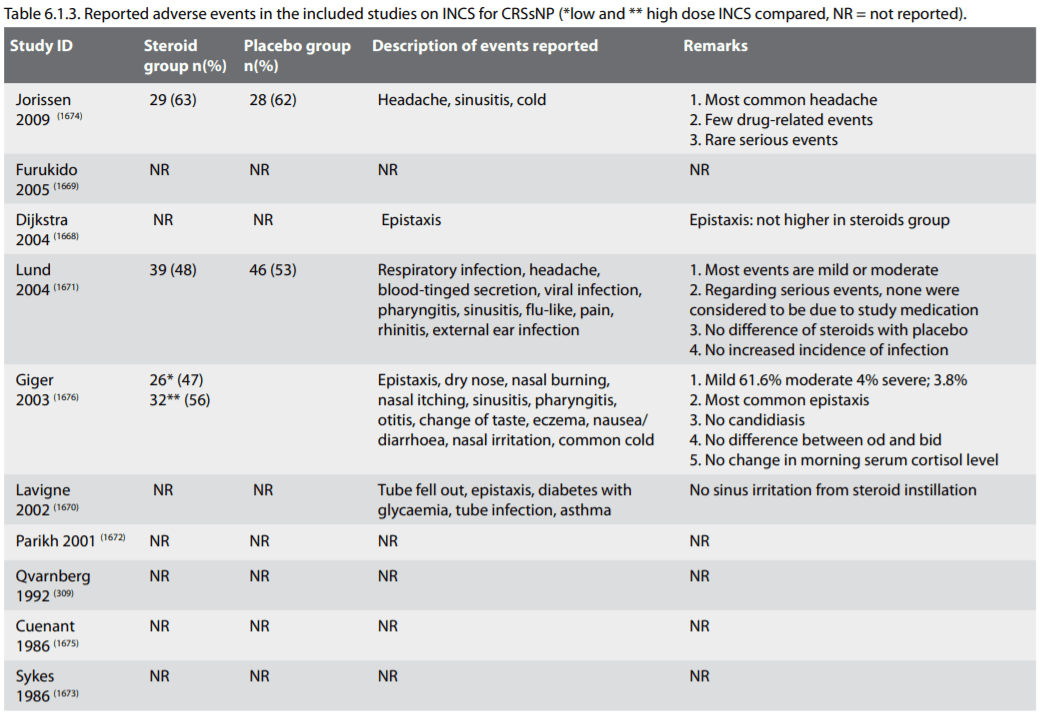
Epistaxis, dry nose, nasal burning and nasal irritation are considered to be drug-related events. It is acknowledged that rare adverse events are possibly not detected in randomised controlled trials (RCTs). However, they were extremely low and there was no difference in adverse events between the study groups and control groups in any trial. Post-market adverse events for intranasal steroid sprays are very low. Minor adverse events from nasal steroid are well tolerated by patients. The amount of benefit clearly outweighs the risk. The reported adverse events from the included studies are summarized in Table 6.1.3.
Epistaxis, dry nose, nasal burning and nasal irritation are considered to be drug-related events. It is acknowledged that rare adverse events are possibly not detected in randomised controlled trials (RCTs). However, they were extremely low and there was no difference in adverse events between the study groups and control groups in any trial. Post-market adverse events for intranasal steroid sprays are very low. Minor adverse events from nasal steroid are well tolerated by patients. The amount of benefit clearly outweighs the risk. The reported adverse events from the included studies are summarized in Table 6.1.3.
6.1.5. Systemic corticosteroid chronic rhinosinusitis without
nasal polyps
6.1.5.1. Introduction
There is limited data showing efficacy of oral corticosteroids in chronic rhinosinusitis without nasal polyps. A systematic review was performed by Lai et al (1678) in 2011. They found 27 clinical human publications on systemic corticosteroid use. Only 1 of these was a prospective trial (case series - level 4 evidence) and no RCTs or controlled cohorts. The remaining publications were 2 retrospective case series and 24 reviews or treatment guidelines. All studies used systemic corticosteroid in conjunction with antibiotics and INCS. Improved subjective and objective outcomes were seen in the 3 studies for CRSsNP (49, 1679, 1680). In Tosca et al. the study population was children with asthma (49). Subramamian et al. had both CRSwNP and CRSsNP and noted that the CRSsNP had better outcomes (1679). Lal et al. noted that the CRSsNP had symptom resolution of 54.9% compared to 51% for the total CRS group (1680).
6.1.5.2. Side-effects of systemic corticosteroid chronic rhinosinusitis without nasal polyps
The side effect profile of corticosteroid use is likely to be similar between CRSsNP and CRSwNP, however, given the relative lack of clinical data (not data against) to support systemic corticosteroid use this risk-benefit ratio may be greater. Please refer to the description of side-effects of systemic corticosteroids from the section on CRSwNP.
6.1.5.3. Evidence based recommendations
There is good evidence that INCS benefit CRSsNP. However, not all author demonstrate this finding. The surgical state of the sinuses treated (i.e. whether the sinuses have been opened and the ability of topical INCS to penetrate into the sinus cavity) appears to have a significant influence on response. The delivery device may be significant but there were not enough studies to come to a conclusion other than technique that deliver more effectively to sinuses are probably more beneficial.
6.1.5.1. Introduction
There is limited data showing efficacy of oral corticosteroids in chronic rhinosinusitis without nasal polyps. A systematic review was performed by Lai et al (1678) in 2011. They found 27 clinical human publications on systemic corticosteroid use. Only 1 of these was a prospective trial (case series - level 4 evidence) and no RCTs or controlled cohorts. The remaining publications were 2 retrospective case series and 24 reviews or treatment guidelines. All studies used systemic corticosteroid in conjunction with antibiotics and INCS. Improved subjective and objective outcomes were seen in the 3 studies for CRSsNP (49, 1679, 1680). In Tosca et al. the study population was children with asthma (49). Subramamian et al. had both CRSwNP and CRSsNP and noted that the CRSsNP had better outcomes (1679). Lal et al. noted that the CRSsNP had symptom resolution of 54.9% compared to 51% for the total CRS group (1680).
6.1.5.2. Side-effects of systemic corticosteroid chronic rhinosinusitis without nasal polyps
The side effect profile of corticosteroid use is likely to be similar between CRSsNP and CRSwNP, however, given the relative lack of clinical data (not data against) to support systemic corticosteroid use this risk-benefit ratio may be greater. Please refer to the description of side-effects of systemic corticosteroids from the section on CRSwNP.
6.1.5.3. Evidence based recommendations
There is good evidence that INCS benefit CRSsNP. However, not all author demonstrate this finding. The surgical state of the sinuses treated (i.e. whether the sinuses have been opened and the ability of topical INCS to penetrate into the sinus cavity) appears to have a significant influence on response. The delivery device may be significant but there were not enough studies to come to a conclusion other than technique that deliver more effectively to sinuses are probably more beneficial.
6.2. Treatment of CRSsNP with antibiotics
6.2.1. Short-term treatment with antibiotics in CRSsNP
No placebo controlled trials exists in short-term systemic antibiotic treatment of CRSsNP
6.2.1.1. Summary of data
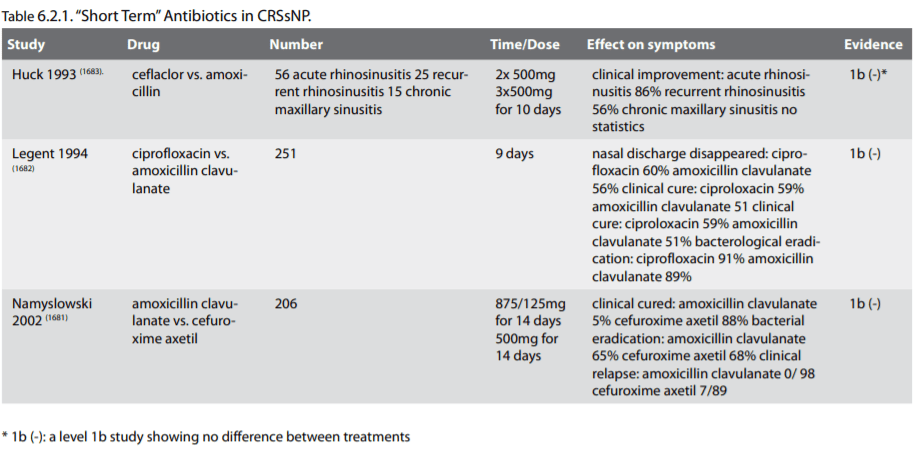
In this review short-term treatment with antibiotics is defined as treatment duration shorter than 4 weeks. There are no placebocontrolled trials available. However three randomised studies were identified, two double-blind and one open comparing the effect of 2 different antibiotics. In a multicentre, open parallel randomised clinical trial 206 adults with exacerbation of CRS were randomised to either amoxicillin/clavulanic acid (875 mg/125 mg b.i.d) or cefuroxime axetil (500 mg b.i.d). Clinical response rate was similar 95 and 88 % respectively. Bacteriological cure rate was 65 and 68 % respectively. Clinical relapse was significantly higher in the cefuroxime group, 8% compared to 0 % in amoxicillin/clavulanic acid group (1681). In a double-blind study 251 CRS patients were randomised to either ciprofloxacin or amoxicillin/clavulanic acid. Clinical cure and bacteriological eradication rate was similar in both groups at approximately 60 % and 90 %. However, at 40 days after treatment cure rate was significantly higher in the ciprofloxacin group and there were more gastrointestinal side effects in the amoxicillin/clavulanic group (1682). In the study by Huck et al comparing cefaclor with amoxicillin only 15 patients with CRS were included, too few to allow statistical analysis (1683).
6.2.1.2. Conclusion
In conclusion, no placebo-controlled studies are available. The 2 studies could not show any difference in short-term outcome comparing different antibiotics. Short-term treatment in CRSsNP is probably only relevant in exacerbations with a positive culture. The present level of evidence is level II. Recommendation B.
6.2.1. Short-term treatment with antibiotics in CRSsNP
No placebo controlled trials exists in short-term systemic antibiotic treatment of CRSsNP
6.2.1.1. Summary of data
In this review short-term treatment with antibiotics is defined as treatment duration shorter than 4 weeks. There are no placebocontrolled trials available. However three randomised studies were identified, two double-blind and one open comparing the effect of 2 different antibiotics. In a multicentre, open parallel randomised clinical trial 206 adults with exacerbation of CRS were randomised to either amoxicillin/clavulanic acid (875 mg/125 mg b.i.d) or cefuroxime axetil (500 mg b.i.d). Clinical response rate was similar 95 and 88 % respectively. Bacteriological cure rate was 65 and 68 % respectively. Clinical relapse was significantly higher in the cefuroxime group, 8% compared to 0 % in amoxicillin/clavulanic acid group (1681). In a double-blind study 251 CRS patients were randomised to either ciprofloxacin or amoxicillin/clavulanic acid. Clinical cure and bacteriological eradication rate was similar in both groups at approximately 60 % and 90 %. However, at 40 days after treatment cure rate was significantly higher in the ciprofloxacin group and there were more gastrointestinal side effects in the amoxicillin/clavulanic group (1682). In the study by Huck et al comparing cefaclor with amoxicillin only 15 patients with CRS were included, too few to allow statistical analysis (1683).
6.2.1.2. Conclusion
In conclusion, no placebo-controlled studies are available. The 2 studies could not show any difference in short-term outcome comparing different antibiotics. Short-term treatment in CRSsNP is probably only relevant in exacerbations with a positive culture. The present level of evidence is level II. Recommendation B.
6.2.2. Long-term treatment with antibiotics in CRSsNP
6.2.2.1. Introduction to long-term treatment with systemic antibiotics in CRS
There has been an increasing interest in the use of macrolides in airway inflammatory disease since the publication of long-term, low-dose erythromycin treatment of diffuse panbronchiolitis (DPB). The treatment changed the 10 years survival rate from 25% to over 90 % and simultaneously cleared the CRS (1684, 1685). Interesting to note is that the effect is seen at lower doses than used to treat infection and that the onset is slow and there is effect in the absence of common pathogens or in the presence of non-sensitive pathogens. Combined with the welldocumented anti-inflammatory effects of macrolides in vitro it has led to the concept of macrolides being immune-modulatory rather than anti-bacterial.
6.2.2.2. Evidence for effect of long-term treatment with macrolides in the lower airways
From the literature it is evident that the pulmonary physicians have been much more successful than the ENT community to initiate Randomised Controlled Trials. In order to understand the potential of macrolide antibiotics to modify the inflammatory response in the airway it is therefore prudent to summarize present evidence from the lower airway. The remarkable effect in diffuse panbronchiolitis patients have already been mentioned (1684, 1686). In CF no less than eight RCTs have showed a beneficiary effect using clarithromycin, (one) or azithromycin, (seven). There are undisputed effects on inflammatory markers, such as IL-8, IL-4, interferon-gamma and TNF-α as well as reducing the rate of exacerbations and reducing decline in lung function (1687-1689). Although not all studies have shown an overall improvement in quality of life it is now a recommended adjunctive treatment in CF.
In asthma, RCT studies using macrolides have shown to reduce airway hyperresponsiveness and to reduce inflammatory mediators in bronchoalvelar lavage such as IL-5, TNF-alpha and IL-12 (1690-1692). More surprisingly roxithromycin therapy reduced markers for eosinophilic activity in aspirin sensitive asthmatics (1693). A subgroup responding well to macrolides are the asthma patients with positive PCR for Chlamydophila pneumoniae or Mycoplasma pneumonia (1690).
Until recently in COPD there were two small (n<100) RCTs showing no effect on health status and exacerbation rate (1694, 1695). However recently a large RCT in COPD (n=1577) using azithromycin for one year, showed a significant effect on time to exacerbation and number of exacerbation as well as improved functioning (1696).
In non-CF bronchiectasis, 3 RCTS have shown reduction in bronchial inflammation and sputum volume, individual studies have also demonstrated pulmonary function improvement and reduction in metacholine induced hyper responsiveness (1697-1699). To sum up, the anti-inflammatory effects of macrolides in the lower airways is clearly demonstrated, especially in a neutrophilic inflammatory- infectious disease, such as CF. One has to bear in mind that a reduced dose was not always used and an added anti-bacterial effect is likely. In asthmatics PCR identification of Chlamydophila or Mycoplasma seems to be one way to identify the responsive phenotype. The case with COPD where 2 small studies showed little or no effect, whereas a large RCT showed effect, is an important reminder that a power analysis is paramount. A similar sized RCT in a defined CRS population would be of great consequence in constituting the care of CRS patients in the future.
6.2.2.3. Long-term treatment with systemic antibiotics in CRSsNP
In CRSsNP there is some evidence to use longterm, low-dose macrolide antibiotics for 12 weeks. Selecting patients with normal serum IgE could improve response rate.
In this review long-term treatment with antibiotics is defined as treatment duration longer than 4 weeks. Although antibiotic treatment is one of the mainstays of CRS treatment the number of placebo controlled trials are limited to two studies. There are a number of open studies using macrolide antibiotics in varying doses, most often about half the daily dose compared to treating acute infections. All studies show a response rate (reduction in symptoms) that varies between 60 and 80 %. Most studies also show a reduction of inflammatory markers and some an increased ciliary beat frequency indicating less sticky secretions (1700-1706). One study compared surgery with 12 weeks of erythromycin. Both treatment modalities improved symptoms significantly, except for nasal volume, which was better in the surgery group (16).
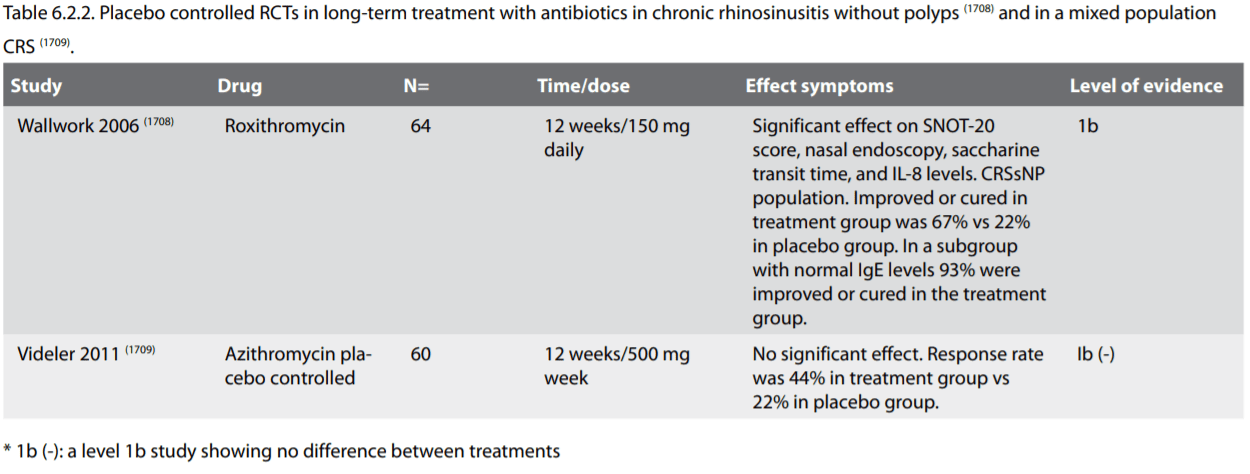
A recent review, June 2011, from the Cochrane Collaboration titled: Systemic antibiotics for chronic rhinosinusitis without nasal polyps (1707) identified only one prospective randomised placebo controlled trial (1708). Recently and not included in the Cochrane review, another randomized controlled study has been published (1709). These two studies represent the only placebo controlled randomised trials available in CRS. The studies investigated the effect of a macrolide antibiotic on the signs, symptoms and quality of life in patients suffering from chronic rhinosinusitis. In both studies the treatment period was 12 weeks. However, whereas the study of Wallwork and co-workers showed a clinical effect of roxithromycin with significant improvements in SNOT-20 score, nasal endoscopy, saccharine transit time, and IL-8 levels in lavage fluid, the study by Videler and co-workers showed that azithromycin lacked efficacy. In the Wallwork study the response rate overall in the treatment group was 67%, compared to 22% in the placebo group whereas in the Videler study it was 44% for azithromycin and 28% for placebo.
Both studies are about the same size 64 vs. 60 patients with CRS included. The inclusion criteria are however different. In the Wallwork study the patients were without polyps, whereas, the Videler study included patients both with and without nasal polyps, in fact a minimum CT score was required (CT scan score ≥5 at worst side according to Mackay-Lund), which suggest a polyposis or hyperplastic sinusitis. In the Wallwork study it was noted that a sub-population of patients with normal IgE levels had a higher response rate to the macrolide treatment than patients with elevated IgE, where most of the non-responders were found. Although not analysed, it is possible that the study population in the Videler study comprised of a higher number of patients with elevated IgE making them less suitable for macrolide treatment as previously described by Suzuki (1710). Higher CT scores are also positively related to elevated IgE levels and eosinophilia (1711). The discrepancy between these two studies highlights the need for matching the right patient with the right treatment. When considering long-term macrolide treatment, a serum IgE is helpful in trying to identify likely responders.
A retrospective analysis compared a mixed CRS population (both with and without polyps) treated with long-term macrolide, azithromycin or clarithromycin or trimethroprimsulfamethoxazole. 76 patients were included, 53% had asthma and all had undergone sinus surgery. Severe nasal polyposis patients were excluded. The mean length of treatment was 189 and 232 day respectively. The response rate was 78% with no difference between the 2 treatment groups. Follow up for 4.7 months in mean after cessation of treatment showed that the improvement was sustained in 68% of patients. Interesting to note, smokers were less likely to respond and there were more allergic patients in the responding group (1712).
6.2.2.1. Introduction to long-term treatment with systemic antibiotics in CRS
There has been an increasing interest in the use of macrolides in airway inflammatory disease since the publication of long-term, low-dose erythromycin treatment of diffuse panbronchiolitis (DPB). The treatment changed the 10 years survival rate from 25% to over 90 % and simultaneously cleared the CRS (1684, 1685). Interesting to note is that the effect is seen at lower doses than used to treat infection and that the onset is slow and there is effect in the absence of common pathogens or in the presence of non-sensitive pathogens. Combined with the welldocumented anti-inflammatory effects of macrolides in vitro it has led to the concept of macrolides being immune-modulatory rather than anti-bacterial.
6.2.2.2. Evidence for effect of long-term treatment with macrolides in the lower airways
From the literature it is evident that the pulmonary physicians have been much more successful than the ENT community to initiate Randomised Controlled Trials. In order to understand the potential of macrolide antibiotics to modify the inflammatory response in the airway it is therefore prudent to summarize present evidence from the lower airway. The remarkable effect in diffuse panbronchiolitis patients have already been mentioned (1684, 1686). In CF no less than eight RCTs have showed a beneficiary effect using clarithromycin, (one) or azithromycin, (seven). There are undisputed effects on inflammatory markers, such as IL-8, IL-4, interferon-gamma and TNF-α as well as reducing the rate of exacerbations and reducing decline in lung function (1687-1689). Although not all studies have shown an overall improvement in quality of life it is now a recommended adjunctive treatment in CF.
In asthma, RCT studies using macrolides have shown to reduce airway hyperresponsiveness and to reduce inflammatory mediators in bronchoalvelar lavage such as IL-5, TNF-alpha and IL-12 (1690-1692). More surprisingly roxithromycin therapy reduced markers for eosinophilic activity in aspirin sensitive asthmatics (1693). A subgroup responding well to macrolides are the asthma patients with positive PCR for Chlamydophila pneumoniae or Mycoplasma pneumonia (1690).
Until recently in COPD there were two small (n<100) RCTs showing no effect on health status and exacerbation rate (1694, 1695). However recently a large RCT in COPD (n=1577) using azithromycin for one year, showed a significant effect on time to exacerbation and number of exacerbation as well as improved functioning (1696).
In non-CF bronchiectasis, 3 RCTS have shown reduction in bronchial inflammation and sputum volume, individual studies have also demonstrated pulmonary function improvement and reduction in metacholine induced hyper responsiveness (1697-1699). To sum up, the anti-inflammatory effects of macrolides in the lower airways is clearly demonstrated, especially in a neutrophilic inflammatory- infectious disease, such as CF. One has to bear in mind that a reduced dose was not always used and an added anti-bacterial effect is likely. In asthmatics PCR identification of Chlamydophila or Mycoplasma seems to be one way to identify the responsive phenotype. The case with COPD where 2 small studies showed little or no effect, whereas a large RCT showed effect, is an important reminder that a power analysis is paramount. A similar sized RCT in a defined CRS population would be of great consequence in constituting the care of CRS patients in the future.
6.2.2.3. Long-term treatment with systemic antibiotics in CRSsNP
In CRSsNP there is some evidence to use longterm, low-dose macrolide antibiotics for 12 weeks. Selecting patients with normal serum IgE could improve response rate.
In this review long-term treatment with antibiotics is defined as treatment duration longer than 4 weeks. Although antibiotic treatment is one of the mainstays of CRS treatment the number of placebo controlled trials are limited to two studies. There are a number of open studies using macrolide antibiotics in varying doses, most often about half the daily dose compared to treating acute infections. All studies show a response rate (reduction in symptoms) that varies between 60 and 80 %. Most studies also show a reduction of inflammatory markers and some an increased ciliary beat frequency indicating less sticky secretions (1700-1706). One study compared surgery with 12 weeks of erythromycin. Both treatment modalities improved symptoms significantly, except for nasal volume, which was better in the surgery group (16).
A recent review, June 2011, from the Cochrane Collaboration titled: Systemic antibiotics for chronic rhinosinusitis without nasal polyps (1707) identified only one prospective randomised placebo controlled trial (1708). Recently and not included in the Cochrane review, another randomized controlled study has been published (1709). These two studies represent the only placebo controlled randomised trials available in CRS. The studies investigated the effect of a macrolide antibiotic on the signs, symptoms and quality of life in patients suffering from chronic rhinosinusitis. In both studies the treatment period was 12 weeks. However, whereas the study of Wallwork and co-workers showed a clinical effect of roxithromycin with significant improvements in SNOT-20 score, nasal endoscopy, saccharine transit time, and IL-8 levels in lavage fluid, the study by Videler and co-workers showed that azithromycin lacked efficacy. In the Wallwork study the response rate overall in the treatment group was 67%, compared to 22% in the placebo group whereas in the Videler study it was 44% for azithromycin and 28% for placebo.
Both studies are about the same size 64 vs. 60 patients with CRS included. The inclusion criteria are however different. In the Wallwork study the patients were without polyps, whereas, the Videler study included patients both with and without nasal polyps, in fact a minimum CT score was required (CT scan score ≥5 at worst side according to Mackay-Lund), which suggest a polyposis or hyperplastic sinusitis. In the Wallwork study it was noted that a sub-population of patients with normal IgE levels had a higher response rate to the macrolide treatment than patients with elevated IgE, where most of the non-responders were found. Although not analysed, it is possible that the study population in the Videler study comprised of a higher number of patients with elevated IgE making them less suitable for macrolide treatment as previously described by Suzuki (1710). Higher CT scores are also positively related to elevated IgE levels and eosinophilia (1711). The discrepancy between these two studies highlights the need for matching the right patient with the right treatment. When considering long-term macrolide treatment, a serum IgE is helpful in trying to identify likely responders.
A retrospective analysis compared a mixed CRS population (both with and without polyps) treated with long-term macrolide, azithromycin or clarithromycin or trimethroprimsulfamethoxazole. 76 patients were included, 53% had asthma and all had undergone sinus surgery. Severe nasal polyposis patients were excluded. The mean length of treatment was 189 and 232 day respectively. The response rate was 78% with no difference between the 2 treatment groups. Follow up for 4.7 months in mean after cessation of treatment showed that the improvement was sustained in 68% of patients. Interesting to note, smokers were less likely to respond and there were more allergic patients in the responding group (1712).
6.2.2.4. Conclusion
The majority of studies have used macrolide antibiotics. A number of open studies using macrolides have shown a response rate of 60-80%. One placebo controlled study using a roxithromycin showed efficacy in patients without polyps. The other placebo controlled azithromycin study had a mixed population of patients with or without polyps and although there were more responders in the treatment group it did not reach significance. Further larger placebo controlled studies in a defined CRS population are warranted. Concerning the open studies one has to be cautious, especially since an intervention is more likely to occur when the patient is suffering from an exacerbation, and as in any cyclic disease an improvement will eventually occur regardless of action taken. Thus, in a study lacking a placebo group, the risk of over-estimating efficacy of the intervention is high.
For now, long-term antibiotic treatment should be reserved for patients where nasal corticosteroids and saline irrigation has failed to reduce symptoms to an acceptable level. Data suggests that the population with high serum IgE are less likely to respond to macrolide treatment and the ones with normal IgE more likely to do so (1713). Future phenotyping may also include PCR for Chlamydiophilia and Mycoplasma although this has not been explored in CRS.
Other choices such as long-term treatment with doxycycline or trimethroprim-sulfamethoxazole could turn out to be promising alternatives and further studies are warranted. Level of evidence for macrolides in all patients with CRSsNP is Ib, and strength of recommendation C, because the two double blind placebo controlled studies are contradictory; indication exist for better efficacy in CRSsNP patients with normal IgE the recommendation A. No RCTs exist for other antibiotics.
6.2.2.5. Adverse events of antibiotic therapy of CRS
6.2.2.5.1. Effects on bacterial resistance.
A concern with long-term antibacterial treatment is the emergence of resistant bacterial strains. Especially when using a low dose not attaining minimal inhibitory concentrations. Data from primary care have shown that increased macrolide prescription in group A streptococci tonsillitis leads to a subsequent increase in resistance, which can reach alarmingly high levels (1714, 1715). However in a tertiary setting, data is sketchy. The study by Videler at al. using azithromycin for 12 weeks, found 3 of 50 cultures with macrolide resistant strains before treatment, and after treatment 4 of 43 cultures with resistant strains (1709). An emerging concern in cystic fibrosis patients is the increasing incidence of infection with the highly pathogenic Mycobacterium abscessus in azithromycin treated patients. The effect is probably due to azithromycin inhibition of autophagic and phagosomal degradation (1716-1718). This has not been reported in CRS patients. In a placebo randomised, doubleblind trial, studying the effect of exposure of oral streptococcal flora of healthy volunteers to azithromycin and clarithromycin, definitive proof that antibiotic use is the single most important driver of antibiotic-resistance was demonstrated. Physicians prescribing antibiotics should take into account these striking ecologic side-effects of antibiotics (1719).
6.2.2.5.2. Other side effects
Well-known side effects of antibiotics includes; gastrointestinal upset, skin rash reversible elevation of liver enzymes. In the study by Videler et al including 78 patients, the investigators found 1 case of muscle ache in the azithroprim group and 2 cases of mild skin rash in the clarithromycin treated patients and no adverse effects in the trimethroprim-sulfamethoxazole group. The study comparing doxycycline treatment for 20 days with methylprednisolone and placebo reported no difference in adverse events in the different groups. However, rare side effects are not picked up in small clinical trials, but rather in national records on side effects. Hearing impairment due to macrolide treatment is a rare side effect but was recorded in a recent large trial in COPD (1696).
6.2.2.5.3. Conclusions on adverse events of antibiotic therapy of CRS
The safety of long-term antibiotic therapy, either azithromycin, clarithromycin or roxithromycin is recognised in patients with CRS, but also due to it's established long-term use in cystic fibrosis. As for doxycycline there is longstanding experience for long-term use in acne and rosacea patients. Trimethroprimsulfamethoxazole has been used long-term in both the paediatric and adult population for treatment of infectious prone patients with certain immune deficiencies as well as urinary tract infections. Drawing on the experience from other areas than CRS, long-term treatment with the mentioned antibiotics is relatively safe. Although one has to bear in mind the interaction between macrolides and drugs such as dicumarol, antiepileptic drugs, terphenadine, methotrexate and antidepressant drugs.
To monitor the risk of the development of resistant bacterial strains, nasal swabs with culture every 3 months during treatment is advisable.
The majority of studies have used macrolide antibiotics. A number of open studies using macrolides have shown a response rate of 60-80%. One placebo controlled study using a roxithromycin showed efficacy in patients without polyps. The other placebo controlled azithromycin study had a mixed population of patients with or without polyps and although there were more responders in the treatment group it did not reach significance. Further larger placebo controlled studies in a defined CRS population are warranted. Concerning the open studies one has to be cautious, especially since an intervention is more likely to occur when the patient is suffering from an exacerbation, and as in any cyclic disease an improvement will eventually occur regardless of action taken. Thus, in a study lacking a placebo group, the risk of over-estimating efficacy of the intervention is high.
For now, long-term antibiotic treatment should be reserved for patients where nasal corticosteroids and saline irrigation has failed to reduce symptoms to an acceptable level. Data suggests that the population with high serum IgE are less likely to respond to macrolide treatment and the ones with normal IgE more likely to do so (1713). Future phenotyping may also include PCR for Chlamydiophilia and Mycoplasma although this has not been explored in CRS.
Other choices such as long-term treatment with doxycycline or trimethroprim-sulfamethoxazole could turn out to be promising alternatives and further studies are warranted. Level of evidence for macrolides in all patients with CRSsNP is Ib, and strength of recommendation C, because the two double blind placebo controlled studies are contradictory; indication exist for better efficacy in CRSsNP patients with normal IgE the recommendation A. No RCTs exist for other antibiotics.
6.2.2.5. Adverse events of antibiotic therapy of CRS
6.2.2.5.1. Effects on bacterial resistance.
A concern with long-term antibacterial treatment is the emergence of resistant bacterial strains. Especially when using a low dose not attaining minimal inhibitory concentrations. Data from primary care have shown that increased macrolide prescription in group A streptococci tonsillitis leads to a subsequent increase in resistance, which can reach alarmingly high levels (1714, 1715). However in a tertiary setting, data is sketchy. The study by Videler at al. using azithromycin for 12 weeks, found 3 of 50 cultures with macrolide resistant strains before treatment, and after treatment 4 of 43 cultures with resistant strains (1709). An emerging concern in cystic fibrosis patients is the increasing incidence of infection with the highly pathogenic Mycobacterium abscessus in azithromycin treated patients. The effect is probably due to azithromycin inhibition of autophagic and phagosomal degradation (1716-1718). This has not been reported in CRS patients. In a placebo randomised, doubleblind trial, studying the effect of exposure of oral streptococcal flora of healthy volunteers to azithromycin and clarithromycin, definitive proof that antibiotic use is the single most important driver of antibiotic-resistance was demonstrated. Physicians prescribing antibiotics should take into account these striking ecologic side-effects of antibiotics (1719).
6.2.2.5.2. Other side effects
Well-known side effects of antibiotics includes; gastrointestinal upset, skin rash reversible elevation of liver enzymes. In the study by Videler et al including 78 patients, the investigators found 1 case of muscle ache in the azithroprim group and 2 cases of mild skin rash in the clarithromycin treated patients and no adverse effects in the trimethroprim-sulfamethoxazole group. The study comparing doxycycline treatment for 20 days with methylprednisolone and placebo reported no difference in adverse events in the different groups. However, rare side effects are not picked up in small clinical trials, but rather in national records on side effects. Hearing impairment due to macrolide treatment is a rare side effect but was recorded in a recent large trial in COPD (1696).
6.2.2.5.3. Conclusions on adverse events of antibiotic therapy of CRS
The safety of long-term antibiotic therapy, either azithromycin, clarithromycin or roxithromycin is recognised in patients with CRS, but also due to it's established long-term use in cystic fibrosis. As for doxycycline there is longstanding experience for long-term use in acne and rosacea patients. Trimethroprimsulfamethoxazole has been used long-term in both the paediatric and adult population for treatment of infectious prone patients with certain immune deficiencies as well as urinary tract infections. Drawing on the experience from other areas than CRS, long-term treatment with the mentioned antibiotics is relatively safe. Although one has to bear in mind the interaction between macrolides and drugs such as dicumarol, antiepileptic drugs, terphenadine, methotrexate and antidepressant drugs.
To monitor the risk of the development of resistant bacterial strains, nasal swabs with culture every 3 months during treatment is advisable.
6.2.3. Treatment with topical antibiotics in CRSsNP
6.2.3.1. Summary of the data
There are three placebo-controlled studies with topical antibiotics and a number of open labelled studies. They open labelled studies show benefit in either signs and or symptoms ranging from 40 to 80% response rate (1455, 1720-1725). A number of different topical solutions have been used with different treatment periods. Any general conclusions from these studies are difficult to draw.
There are 3 placebo-controlled trials with topical antibiotics in CRS. None of them showed any additive effect compared to saline.
However the three placebo-controlled studies are all negative. A study from 1986 where dexamethasone, neomycine and tramazoline were compared with dexamethasone without neomycine and a placebo group with vehicle alone showed no additive effect of neomycin both the group with dexamethasone alone and with the addition of neomycin were superior to placebo (1673). Another placebo controlled trial by Desrosiers et al. investigated 20 patients in a randomized, double-blind trial of tobramycin-saline solution or saline-only, administered thrice daily by means of a nebulizer for 4 weeks, followed by a 4-week observation period. Both patient groups experienced improvement in signs and symptoms but the addition of tobramycin appears of no benefit (1726). Thirdly a study by Videler et al investigated the effect of nasal irrigation with bacitracin/colimycin or placebo in a randomised, double blind, cross-over study in 14 patients with recalcitrant CRS in spite of surgery. Both groups improved and there was no difference in SF-36 and endoscopic appearance (1727). Chiu et al showed in a rabbit model with Pseudomonas sinus infection that increasing concentrations of topical tobramycin resulted in the eradication of viable bacteria within the lumen of the sinus but did not eradicate Pseudomonas attached to the mucosa (1728).
6.2.3.1. Summary of the data
There are three placebo-controlled studies with topical antibiotics and a number of open labelled studies. They open labelled studies show benefit in either signs and or symptoms ranging from 40 to 80% response rate (1455, 1720-1725). A number of different topical solutions have been used with different treatment periods. Any general conclusions from these studies are difficult to draw.
There are 3 placebo-controlled trials with topical antibiotics in CRS. None of them showed any additive effect compared to saline.
However the three placebo-controlled studies are all negative. A study from 1986 where dexamethasone, neomycine and tramazoline were compared with dexamethasone without neomycine and a placebo group with vehicle alone showed no additive effect of neomycin both the group with dexamethasone alone and with the addition of neomycin were superior to placebo (1673). Another placebo controlled trial by Desrosiers et al. investigated 20 patients in a randomized, double-blind trial of tobramycin-saline solution or saline-only, administered thrice daily by means of a nebulizer for 4 weeks, followed by a 4-week observation period. Both patient groups experienced improvement in signs and symptoms but the addition of tobramycin appears of no benefit (1726). Thirdly a study by Videler et al investigated the effect of nasal irrigation with bacitracin/colimycin or placebo in a randomised, double blind, cross-over study in 14 patients with recalcitrant CRS in spite of surgery. Both groups improved and there was no difference in SF-36 and endoscopic appearance (1727). Chiu et al showed in a rabbit model with Pseudomonas sinus infection that increasing concentrations of topical tobramycin resulted in the eradication of viable bacteria within the lumen of the sinus but did not eradicate Pseudomonas attached to the mucosa (1728).

6.2.3.2. Conclusion concerning the use of topical antibiotics
in CRS
There is low level of evidence for the efficacy of topical antibacterial therapy in seven uncontrolled trials. However 3 placebo controlled trials failed to show any additive effect of topical antibiotics as compared to saline alone. Topical antibacterial therapy cannot be recommended in the treatment of CRS. Level of evidence Ib, grade of recommendation A.
6.2.3.3. Adverse events of topical antibiotic spray
Not all studies mention side effects but the most common side effects seems to be intra-nasal stinging, burning sensation, moderate pain, throat irritation, cough and dry skin. Topical antibiotics not being registered as drugs makes reports on side effects sketchy.
There is low level of evidence for the efficacy of topical antibacterial therapy in seven uncontrolled trials. However 3 placebo controlled trials failed to show any additive effect of topical antibiotics as compared to saline alone. Topical antibacterial therapy cannot be recommended in the treatment of CRS. Level of evidence Ib, grade of recommendation A.
6.2.3.3. Adverse events of topical antibiotic spray
Not all studies mention side effects but the most common side effects seems to be intra-nasal stinging, burning sensation, moderate pain, throat irritation, cough and dry skin. Topical antibiotics not being registered as drugs makes reports on side effects sketchy.
6.3. Other medical management in CRSsNP
6.3.1. Summary
This chapter deals with medical therapies of CRSsNP in adults except antibiotics and glucocorticoids. For medical treatment of acute rhinosinusitis and in paediatric rhinosinusitis, please refer to the according chapters. No RCT for the treatment of CRSsNP in adults were identified for antihistamines, mucolytics and expectorants, homeopathic remedies, proton pump inhibitors, surfactants including baby shampoo or nasal decongestants. These treatment modalities are not recommended. No benefit was found in randomized controlled trials or systemic reviews for antimycotics, herbal medicines, or probiotics, which are also not recommended for the treatment of CRSsNP in adults. Based on the results of 1 RCT, bacterial lysate treatment may be considered as an adjunct to standard medical treatment in adults with CRSsNP. One Cochrane review and 2 RCTs indicate a beneficial effect of nasal douches in CRSsNP in adults.
6.3.2. Antimycotics
One trial with nasal amphotericin B treatment was explicitly performed in 64 CRS-patients without polyps (711). Following inclusion, patients were randomized to either 20mg/day amphotericin B or a yellowish solution without amphotericin administered in 500 ml saline solution with a pulsatile irrigator. If the type of therapy was concealed to the investigators is not reported. Main outcome parameter was the sum score of the Rhinosinusitis Outcome Measures 31 questionnaire. Secondary endpoints included a nasal endoscopy score and pre- and post-treatment fungal cultures. Symptom scores were significantly lower in the amphotericin treated patients after 2 weeks treatment (p=0.018), but not after 4 weeks treatment (p=0.091). Endoscopy scores and fungal culture rates did not significantly differ between groups. Based on current data, nasal amphotericin B treatment in CRSsNP is not recommended (grade of recommendation A).
6.3.3. Bacterial Lysates
Bacterial lysates enhance Th1-skewed immune responses and dendritic cell maturation via activation of toll like receptors (1729, 1730). Several trials on the preventive effect of immunostimulants including bacterial lysates on recurrent respiratory infections mainly in children were identified, however, only 1 bacterial lysate trial particularly assessed the effect on chronic rhinosinusitis in adults. In a multicentre randomized doubleblind study, 284 patients with chronic purulent sinusitis were treated with the oral bacterial lysate Broncho Vaxom (OM-85 BV) or placebo in addition to standard therapy (antibiotics, mucolytics, inhalants). Treatment lasted for three ten-day periods in three consecutive months. At the start and during the therapy as well as after six months, symptoms were assessed on the basis of a scoring system and the X-rays of the nasal sinuses evaluated. During the course of therapy and the follow-up period, improvement of the major symptoms headache, purulent nasal discharge, cough, and expectoration was statistically significant in the immunostimulant group as compared with the placebo group, objective evidence being provided by the X-ray examinations and the number of reinfections during the period of observation (1731). Based on the results of 1 RCT, oral OM-85 BV treatment may be considered as an adjunct to standard medical treatment in adults with CRSsNP (grade of recommendation A).
6.3.4. Herbal medicines and homeopathic drugs
Phytotherapy is the use of plants or herbs to treat diseases. A huge range of preparations, most of them not yet subjected to clinical trials and some with unknown ingredients, are marketed over the counter in Europe. Homeopathy is a system of therapeutics founded by Samuel Hahnemann (1755-1843), based on the Law of Similars where "like cures like". Diseases are treated by highly diluted substances that cause, in healthy persons, symptoms like those of the disease to be treated. Herbal and homeopathic drug use is subjected to great regional differences. Alternative treatment modalities are used by 15-50% of rhinosinusitis patients (1732-1734). Guo and co-authors reviewed randomized clinical trials (RCTs) testing a herbal preparation, as sole or adjunctive treatment, administered systemically or topically, against a control intervention (placebo or no treatment), in patients with acute or chronic rhinosinusitis (1735).The authors found no evidence that any herbal medicines are beneficial in the treatment of CRSsNP. Alcoholic extracts of pelargonii radix are marketed since decades as a treatment for upper and lower respiratory tract infections. In a recent Cochrane report on P. sidoides extracts and tablets, no trials on CRSsNP fulfilled the inclusion criteria (336).
No RCT on homeopathic treatment of CRSsNP could be identified. Based on current data, herbal medicines and homeopathic remedies are not recommended for the treatment of CRSsNP (grade of evidence D).
6.3.5. Nasal irrigation
Isotonic or hypertonic saline solutions delivered by bottle, spray, pump or nebuliser are frequently used in the treatment of sinus disease, mainly as a supplement to other therapies. Sinus penetration of irrigation fluids differs in patients with and without previous sinus surgery (1663) and depends on the application mode (1661,1662). Nasal saline irrigations were judged beneficial in the treatment of the symptoms of chronic rhinosinusitis when used as the sole modality of treatment in a Cochrane report (1736). However, in this evaluation children were also included and no clear separation between CRSwNP and CRSsNP was reported. Moreover, it remained unclear, if patients had undergone previous sinus surgery
In a community based, randomized, controled trial, Pynnonen and co-workers compared isotonic nasal saline spray and isotonic nasal saline douches in 127 adult patients with CRS without recent sinus surgery. Outcome parameters included change in symptom severity measured by mean 20-Item Sino-Nasal Outcome Test (SNOT-20) score; change in symptom frequency measured with a global question; and change in medication use. Outcomes were measured at 2, 4, and 8 weeks after randomization. All outcome parameters were significantly better in the nasal douches group than in the nasal spray group (1737).
The value of nasal douching following sinus surgery was assessed in an intra-individual, single blinded randomised controlled trial. Nasal douches were used by 22 patients following sinus surgery in one side of the nasal cavity, three times per day for 6 weeks. The opposite nasal cavity was not irrigated. Presence of adhesions, polyps, crusting, discharge or oedema was assessed 3 weeks and 3 months postoperatively. At 3 weeks, nasal saline douching improved the presence of discharge and oedema, but had no effect on adhesions or crusting. At 3 months, no significant differences between douched and non-douched nasal cavities were observed (1738). Thorough cleaning of irrigation devices is required to prevent bacterial contamination, however, no sinus infection due to irrigation device contamination has yet been reported (1739-1741). Based on current data, nasal douches are recommended for CRSsNP in adults without recent sinus surgery and in the post sinus surgery setting (grade of recommendation A).
6.3.1. Summary
This chapter deals with medical therapies of CRSsNP in adults except antibiotics and glucocorticoids. For medical treatment of acute rhinosinusitis and in paediatric rhinosinusitis, please refer to the according chapters. No RCT for the treatment of CRSsNP in adults were identified for antihistamines, mucolytics and expectorants, homeopathic remedies, proton pump inhibitors, surfactants including baby shampoo or nasal decongestants. These treatment modalities are not recommended. No benefit was found in randomized controlled trials or systemic reviews for antimycotics, herbal medicines, or probiotics, which are also not recommended for the treatment of CRSsNP in adults. Based on the results of 1 RCT, bacterial lysate treatment may be considered as an adjunct to standard medical treatment in adults with CRSsNP. One Cochrane review and 2 RCTs indicate a beneficial effect of nasal douches in CRSsNP in adults.
6.3.2. Antimycotics
One trial with nasal amphotericin B treatment was explicitly performed in 64 CRS-patients without polyps (711). Following inclusion, patients were randomized to either 20mg/day amphotericin B or a yellowish solution without amphotericin administered in 500 ml saline solution with a pulsatile irrigator. If the type of therapy was concealed to the investigators is not reported. Main outcome parameter was the sum score of the Rhinosinusitis Outcome Measures 31 questionnaire. Secondary endpoints included a nasal endoscopy score and pre- and post-treatment fungal cultures. Symptom scores were significantly lower in the amphotericin treated patients after 2 weeks treatment (p=0.018), but not after 4 weeks treatment (p=0.091). Endoscopy scores and fungal culture rates did not significantly differ between groups. Based on current data, nasal amphotericin B treatment in CRSsNP is not recommended (grade of recommendation A).
6.3.3. Bacterial Lysates
Bacterial lysates enhance Th1-skewed immune responses and dendritic cell maturation via activation of toll like receptors (1729, 1730). Several trials on the preventive effect of immunostimulants including bacterial lysates on recurrent respiratory infections mainly in children were identified, however, only 1 bacterial lysate trial particularly assessed the effect on chronic rhinosinusitis in adults. In a multicentre randomized doubleblind study, 284 patients with chronic purulent sinusitis were treated with the oral bacterial lysate Broncho Vaxom (OM-85 BV) or placebo in addition to standard therapy (antibiotics, mucolytics, inhalants). Treatment lasted for three ten-day periods in three consecutive months. At the start and during the therapy as well as after six months, symptoms were assessed on the basis of a scoring system and the X-rays of the nasal sinuses evaluated. During the course of therapy and the follow-up period, improvement of the major symptoms headache, purulent nasal discharge, cough, and expectoration was statistically significant in the immunostimulant group as compared with the placebo group, objective evidence being provided by the X-ray examinations and the number of reinfections during the period of observation (1731). Based on the results of 1 RCT, oral OM-85 BV treatment may be considered as an adjunct to standard medical treatment in adults with CRSsNP (grade of recommendation A).
6.3.4. Herbal medicines and homeopathic drugs
Phytotherapy is the use of plants or herbs to treat diseases. A huge range of preparations, most of them not yet subjected to clinical trials and some with unknown ingredients, are marketed over the counter in Europe. Homeopathy is a system of therapeutics founded by Samuel Hahnemann (1755-1843), based on the Law of Similars where "like cures like". Diseases are treated by highly diluted substances that cause, in healthy persons, symptoms like those of the disease to be treated. Herbal and homeopathic drug use is subjected to great regional differences. Alternative treatment modalities are used by 15-50% of rhinosinusitis patients (1732-1734). Guo and co-authors reviewed randomized clinical trials (RCTs) testing a herbal preparation, as sole or adjunctive treatment, administered systemically or topically, against a control intervention (placebo or no treatment), in patients with acute or chronic rhinosinusitis (1735).The authors found no evidence that any herbal medicines are beneficial in the treatment of CRSsNP. Alcoholic extracts of pelargonii radix are marketed since decades as a treatment for upper and lower respiratory tract infections. In a recent Cochrane report on P. sidoides extracts and tablets, no trials on CRSsNP fulfilled the inclusion criteria (336).
No RCT on homeopathic treatment of CRSsNP could be identified. Based on current data, herbal medicines and homeopathic remedies are not recommended for the treatment of CRSsNP (grade of evidence D).
6.3.5. Nasal irrigation
Isotonic or hypertonic saline solutions delivered by bottle, spray, pump or nebuliser are frequently used in the treatment of sinus disease, mainly as a supplement to other therapies. Sinus penetration of irrigation fluids differs in patients with and without previous sinus surgery (1663) and depends on the application mode (1661,1662). Nasal saline irrigations were judged beneficial in the treatment of the symptoms of chronic rhinosinusitis when used as the sole modality of treatment in a Cochrane report (1736). However, in this evaluation children were also included and no clear separation between CRSwNP and CRSsNP was reported. Moreover, it remained unclear, if patients had undergone previous sinus surgery
In a community based, randomized, controled trial, Pynnonen and co-workers compared isotonic nasal saline spray and isotonic nasal saline douches in 127 adult patients with CRS without recent sinus surgery. Outcome parameters included change in symptom severity measured by mean 20-Item Sino-Nasal Outcome Test (SNOT-20) score; change in symptom frequency measured with a global question; and change in medication use. Outcomes were measured at 2, 4, and 8 weeks after randomization. All outcome parameters were significantly better in the nasal douches group than in the nasal spray group (1737).
The value of nasal douching following sinus surgery was assessed in an intra-individual, single blinded randomised controlled trial. Nasal douches were used by 22 patients following sinus surgery in one side of the nasal cavity, three times per day for 6 weeks. The opposite nasal cavity was not irrigated. Presence of adhesions, polyps, crusting, discharge or oedema was assessed 3 weeks and 3 months postoperatively. At 3 weeks, nasal saline douching improved the presence of discharge and oedema, but had no effect on adhesions or crusting. At 3 months, no significant differences between douched and non-douched nasal cavities were observed (1738). Thorough cleaning of irrigation devices is required to prevent bacterial contamination, however, no sinus infection due to irrigation device contamination has yet been reported (1739-1741). Based on current data, nasal douches are recommended for CRSsNP in adults without recent sinus surgery and in the post sinus surgery setting (grade of recommendation A).
6.3.6. Additions to nasal irrigation
Sodium hypochlorite (NaOCl) is a well-known bleaching and desinfecting agent that has been found to be effective against several organisms including S. aureus and P. aeruginosa. Nasal irrigation with 0.05% NaOCl solution in saline was significantly more effective than saline alone in the treatment of S. aureus positive CRS patients in a study where patients used saline irrigation for 3 months and afterwards saline irrigation with 0.05% NaOCl solution (1742) (Level of evidence IIb). Xylitol has been shown to effect ASL ionic composition in vitro and to reduce nasal bacterial carriage, otitis media, and dental caries in vivo. Xylitol in water is a well-tolerated agent for sinonasal irrigation. Xylitol irrigations result in greater improvement of symptoms of chronic rhinosinusitis as compared to saline irrigation (1743) (level of evidence 1b). Biofilms are considered to play a pathophysiological role in CRS. Data on the impact of biofilms on sinus surgery outcomes are conflicting (693, 1744). Surfactants reduce water surface tension and may help to dissolve biofilms (1745). No RCT of surfactants in the treatment of CRSsNP was identified. Baby shampoo contains several surface active agents. Nasal irrigations containing Johnson's Baby Shampoo were tested in a non-randomized, open-label trial in 15 CRS patients for 4 weeks. Concomitant medications included antibiotics and oral prednisolone. Subjective improvement was observed in 46% of the patients (1746) (level of evidence III).
Current data do support the use of xylitol (recommendation A) or sodium hypochlorite nasal irrigations (grade of recommendations B) but not irrigations containing baby shampoo in CRS patients (grade of recommendation D).
6.3.7. Probiotics
Probiotics are living microorganisms that benefit the health of the host by conditioning the intestinal microenvironment. Supplementation with probiotics may alter intestinal microflora and promote Th1 responses by activating interferon gamma, interleukins 12 and 18. In a randomized, double-blinded, placebo-controlled trial, 77 patients with chronic rhinosinusitis received either oral probiotic Lactobacillus rhamnosus R0011 strain (500 million active cells/tablet twice daily, n=39) or oral placebo (n =38) for 4 weeks. The main study endpoint was the change in the SNOT-20 score. Secondary outcome parameters included a symptom frequency score and a medication score. No significant differences were found between the probiotic and the placebo group in their changes in SNOT-20 scores from baseline to 4 weeks (p=0.79) or from baseline to 8 weeks (p=0.23) (1747). Current data do not support probiotic treatment in CRSsNP (grade of recommendation A).
6.3.8. Proton Pump inhibitors
Extraesophageal reflux has been supposed a possible cause of CRS (1748), however clinical data on the frequency of extraoesophageal reflux in patients with rhinosinusitis do not support this link (1749, 1750). No RCT on proton pump inhibitors in the treatment of CRSsNP in adults was identified. In one uncontrolled trial in 11 adult CRS patients with abnormal pHmonitoring, omeprazole 20mg daily for 12 weeks led to modest symptom improvement (1751). Proton pump inhibitors frequently cause gastrointestinal symptoms and increase the risk to acquire pneumonia in children and respiratory infection in adults (1752, 1753). Current data do not provide sufficient evidence for proton pump inhibitor treatment for CRSsNP in adults (grade of recommendation D).
Sodium hypochlorite (NaOCl) is a well-known bleaching and desinfecting agent that has been found to be effective against several organisms including S. aureus and P. aeruginosa. Nasal irrigation with 0.05% NaOCl solution in saline was significantly more effective than saline alone in the treatment of S. aureus positive CRS patients in a study where patients used saline irrigation for 3 months and afterwards saline irrigation with 0.05% NaOCl solution (1742) (Level of evidence IIb). Xylitol has been shown to effect ASL ionic composition in vitro and to reduce nasal bacterial carriage, otitis media, and dental caries in vivo. Xylitol in water is a well-tolerated agent for sinonasal irrigation. Xylitol irrigations result in greater improvement of symptoms of chronic rhinosinusitis as compared to saline irrigation (1743) (level of evidence 1b). Biofilms are considered to play a pathophysiological role in CRS. Data on the impact of biofilms on sinus surgery outcomes are conflicting (693, 1744). Surfactants reduce water surface tension and may help to dissolve biofilms (1745). No RCT of surfactants in the treatment of CRSsNP was identified. Baby shampoo contains several surface active agents. Nasal irrigations containing Johnson's Baby Shampoo were tested in a non-randomized, open-label trial in 15 CRS patients for 4 weeks. Concomitant medications included antibiotics and oral prednisolone. Subjective improvement was observed in 46% of the patients (1746) (level of evidence III).
Current data do support the use of xylitol (recommendation A) or sodium hypochlorite nasal irrigations (grade of recommendations B) but not irrigations containing baby shampoo in CRS patients (grade of recommendation D).
6.3.7. Probiotics
Probiotics are living microorganisms that benefit the health of the host by conditioning the intestinal microenvironment. Supplementation with probiotics may alter intestinal microflora and promote Th1 responses by activating interferon gamma, interleukins 12 and 18. In a randomized, double-blinded, placebo-controlled trial, 77 patients with chronic rhinosinusitis received either oral probiotic Lactobacillus rhamnosus R0011 strain (500 million active cells/tablet twice daily, n=39) or oral placebo (n =38) for 4 weeks. The main study endpoint was the change in the SNOT-20 score. Secondary outcome parameters included a symptom frequency score and a medication score. No significant differences were found between the probiotic and the placebo group in their changes in SNOT-20 scores from baseline to 4 weeks (p=0.79) or from baseline to 8 weeks (p=0.23) (1747). Current data do not support probiotic treatment in CRSsNP (grade of recommendation A).
6.3.8. Proton Pump inhibitors
Extraesophageal reflux has been supposed a possible cause of CRS (1748), however clinical data on the frequency of extraoesophageal reflux in patients with rhinosinusitis do not support this link (1749, 1750). No RCT on proton pump inhibitors in the treatment of CRSsNP in adults was identified. In one uncontrolled trial in 11 adult CRS patients with abnormal pHmonitoring, omeprazole 20mg daily for 12 weeks led to modest symptom improvement (1751). Proton pump inhibitors frequently cause gastrointestinal symptoms and increase the risk to acquire pneumonia in children and respiratory infection in adults (1752, 1753). Current data do not provide sufficient evidence for proton pump inhibitor treatment for CRSsNP in adults (grade of recommendation D).
6.4. Evidence based surgery for CRSsNP
6.4.1. Summary
Although trials providing high level evidence are missing, a number of large, well organised prospective studies has shown that endoscopic sinus surgery (ESS) is safe and effective in managing patients with CRS without NP when medical treatment has failed. ESS is more likely to be effective in managing nasal obstruction and facial pain than postnasal drip or Hyposmia and is associated in significant improvements in generic as well as disease specific quality of life outcomes. Middle meatal antrostomy as opposed to simple uncinectomy and (targeted) partial removal of the middle turbinate may be associated with improved endoscopic and radiological outcomes but not subjective improvements.
6.4.2. Introduction
Surgery is an imprecise art, and surgeons have traditionally had to make decisions with limited facts: Unfortunately, a look at the past will reveal a surgical landscape virtually "littered " with procedures and interventions that have now been abandoned and are deemed useless and even harmful. Evidence-based surgery emphasizes the need to evaluate adequately the efficacy of surgical interventions before accepting them as standard. Essential for evidence-based surgery is a clear definition of the disease and standardized outcome measures.
6.4.3. Evidence based surgical treatment of rhinosinusitis
Evidence based medicine does not always have to be based on randomized controlled trials (RCT). In surgery (just as in parachuting (1754)) it is often not ethical or possible to do RCTs; however, the fact remains that we need to evaluate the available evidence to prevent us from giving our patients ineffective or even harmful treatments. Evaluation of the available evidence is not always easy: There are a number of potential biases in all types of medical research (expectancy bias/patients expectations from treatment, variations between patients/ selection bias, co-intervention and timing bias, publication bias and withdrawal bias). Surgical studies introduce additional types of bias, including the lack of patient blinding to the surgical intervention and performance bias (procedures or interventions are not executed in a uniform way – any one surgeon may do the same procedure in a different way from day to day, and that is even more true between different surgeons). Despite these difficulties, studies are being performed, and sinus surgeons should critically evaluate published evidence and adjust their practices accordingly. We will attempt in this article to assess evidence on surgery for CRSsNP or CRSwNP, taking into account however, that many studies included patients with CRS with and without nasal polyps.
6.4.4. Functional endoscopic sinus surgery in CRSsNP
Large prospective studies and case series have shown that endoscopic sinus surgery is effective and safe for the management of patients of CRS without NP who have failed medical treatment
6.4.4.1. Randomised controlled trials
The "holy grail" of evidence-based medicine is the Randomised Controlled trial (RCT). However, the search for such studies is not always successful, and in the meanwhile, surgeons have to practice with the (best) available evidence. The Cochrane collaboration re-assessed and revised in 2009 (1755) the evidence for surgery in CRS: they screened 2323 studies and found 6 randomised control trials: Using strict methodological quality inclusion criteria they excluded 3 of these studies and examined the three remaining RCTs: the first one, a University of London thesis written by Fairley in 1993 (1153) compared 12 patients with CRS undergoing endoscopic middle meatal antrostomy with 17 patients who undergoing conventional intranasal inferior meatal antrostomy. The study found symptom improvement in both groups but no differences between groups; one year follow-up data were available for 11 patients and 9 patients respectively, a sample size the author acknowledges as being too small to exclude a type II error. The second study from 1997 (1756) assessed patients with isolated maxillary sinusitis and compared symptom improvement after ESS and after saline rinses among patients randomized before antibiotics were administered. The third study by Ragab et al. from 2004 (16) was the most relevant of the three as it randomized 90 patients with CRS with and without NP in medical (long term antibiotics) or surgical (ESS) management and assessed both objective (endoscopic scores, nitric oxide, PNIF and saccharine clearance time) and patient reported outcomes (symptoms VAS, SF36 and SNOT 20 scores). It found that both treatments significantly improved almost all the subjective and objective parameters of CRS (P <.01), with no significant difference being found between the medical and surgical groups, except for the total nasal volume, in which the surgical treatment demonstrated greater changes. The effect of surgery was the same in both CRS groups (with and without NP). However, the surgical group received only 2 weeks of erythromycin after surgery while the medical group received 3 months of erythromycin after randomization. However, there was no placebo group, which somewhat reduces the importance of findings.
The Cochrane collaboration, using data from these 212 patients, stated that "(ESS) has not been demonstrated to confer additional benefit to that obtained by medical treatment with or without antral irrigation in relieving the symptoms of chronic rhinosinusitis". However, our impression is that there was simply insufficient evidence for any comment about the value of ESS compared with medical treatment based on these three studies. The first study included in the Cochrane review (1153) did not compare ESS with medical treatment, and the other two studies (16, 1756) did not analyse ESS results among patients who failed medical treatment, including antibiotic therapy. Indeed, current thinking precludes that sinus surgery must be always preceded and/or followed by various forms of medical treatment. This, together with the fact that surgery is often suggested for patients who fail medical therapy render comparisons of medical treatment with surgery difficult (Table 6.4.1).
6.4.1. Summary
Although trials providing high level evidence are missing, a number of large, well organised prospective studies has shown that endoscopic sinus surgery (ESS) is safe and effective in managing patients with CRS without NP when medical treatment has failed. ESS is more likely to be effective in managing nasal obstruction and facial pain than postnasal drip or Hyposmia and is associated in significant improvements in generic as well as disease specific quality of life outcomes. Middle meatal antrostomy as opposed to simple uncinectomy and (targeted) partial removal of the middle turbinate may be associated with improved endoscopic and radiological outcomes but not subjective improvements.
6.4.2. Introduction
Surgery is an imprecise art, and surgeons have traditionally had to make decisions with limited facts: Unfortunately, a look at the past will reveal a surgical landscape virtually "littered " with procedures and interventions that have now been abandoned and are deemed useless and even harmful. Evidence-based surgery emphasizes the need to evaluate adequately the efficacy of surgical interventions before accepting them as standard. Essential for evidence-based surgery is a clear definition of the disease and standardized outcome measures.
6.4.3. Evidence based surgical treatment of rhinosinusitis
Evidence based medicine does not always have to be based on randomized controlled trials (RCT). In surgery (just as in parachuting (1754)) it is often not ethical or possible to do RCTs; however, the fact remains that we need to evaluate the available evidence to prevent us from giving our patients ineffective or even harmful treatments. Evaluation of the available evidence is not always easy: There are a number of potential biases in all types of medical research (expectancy bias/patients expectations from treatment, variations between patients/ selection bias, co-intervention and timing bias, publication bias and withdrawal bias). Surgical studies introduce additional types of bias, including the lack of patient blinding to the surgical intervention and performance bias (procedures or interventions are not executed in a uniform way – any one surgeon may do the same procedure in a different way from day to day, and that is even more true between different surgeons). Despite these difficulties, studies are being performed, and sinus surgeons should critically evaluate published evidence and adjust their practices accordingly. We will attempt in this article to assess evidence on surgery for CRSsNP or CRSwNP, taking into account however, that many studies included patients with CRS with and without nasal polyps.
6.4.4. Functional endoscopic sinus surgery in CRSsNP
Large prospective studies and case series have shown that endoscopic sinus surgery is effective and safe for the management of patients of CRS without NP who have failed medical treatment
6.4.4.1. Randomised controlled trials
The "holy grail" of evidence-based medicine is the Randomised Controlled trial (RCT). However, the search for such studies is not always successful, and in the meanwhile, surgeons have to practice with the (best) available evidence. The Cochrane collaboration re-assessed and revised in 2009 (1755) the evidence for surgery in CRS: they screened 2323 studies and found 6 randomised control trials: Using strict methodological quality inclusion criteria they excluded 3 of these studies and examined the three remaining RCTs: the first one, a University of London thesis written by Fairley in 1993 (1153) compared 12 patients with CRS undergoing endoscopic middle meatal antrostomy with 17 patients who undergoing conventional intranasal inferior meatal antrostomy. The study found symptom improvement in both groups but no differences between groups; one year follow-up data were available for 11 patients and 9 patients respectively, a sample size the author acknowledges as being too small to exclude a type II error. The second study from 1997 (1756) assessed patients with isolated maxillary sinusitis and compared symptom improvement after ESS and after saline rinses among patients randomized before antibiotics were administered. The third study by Ragab et al. from 2004 (16) was the most relevant of the three as it randomized 90 patients with CRS with and without NP in medical (long term antibiotics) or surgical (ESS) management and assessed both objective (endoscopic scores, nitric oxide, PNIF and saccharine clearance time) and patient reported outcomes (symptoms VAS, SF36 and SNOT 20 scores). It found that both treatments significantly improved almost all the subjective and objective parameters of CRS (P <.01), with no significant difference being found between the medical and surgical groups, except for the total nasal volume, in which the surgical treatment demonstrated greater changes. The effect of surgery was the same in both CRS groups (with and without NP). However, the surgical group received only 2 weeks of erythromycin after surgery while the medical group received 3 months of erythromycin after randomization. However, there was no placebo group, which somewhat reduces the importance of findings.
The Cochrane collaboration, using data from these 212 patients, stated that "(ESS) has not been demonstrated to confer additional benefit to that obtained by medical treatment with or without antral irrigation in relieving the symptoms of chronic rhinosinusitis". However, our impression is that there was simply insufficient evidence for any comment about the value of ESS compared with medical treatment based on these three studies. The first study included in the Cochrane review (1153) did not compare ESS with medical treatment, and the other two studies (16, 1756) did not analyse ESS results among patients who failed medical treatment, including antibiotic therapy. Indeed, current thinking precludes that sinus surgery must be always preceded and/or followed by various forms of medical treatment. This, together with the fact that surgery is often suggested for patients who fail medical therapy render comparisons of medical treatment with surgery difficult (Table 6.4.1).
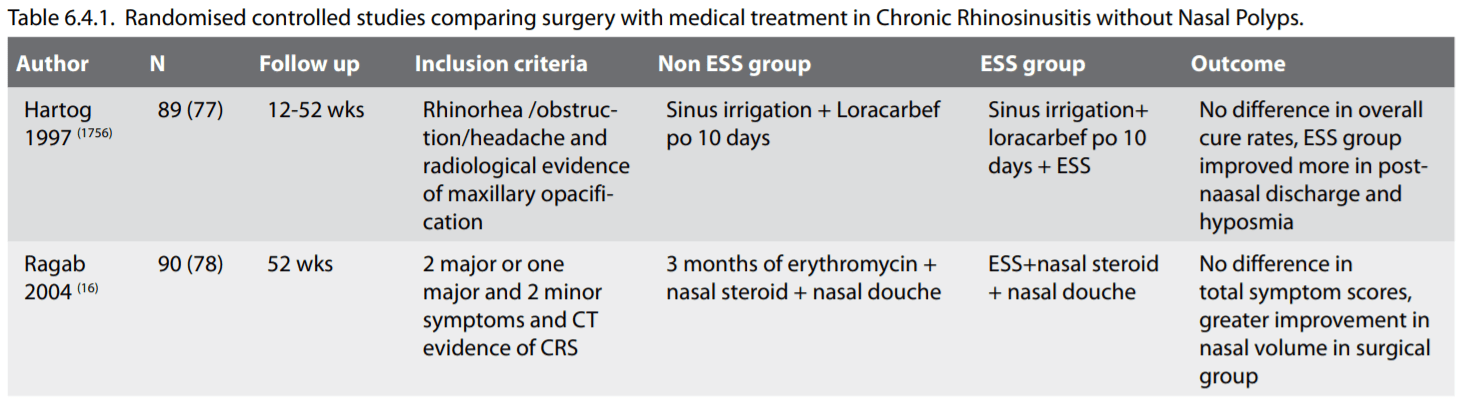
6.4.4.2. Case series, case-control and cohort studies
RCTs in surgery are notoriously difficult to organise and (as in the case of truly blind "sham" studies), potentially unethical. If these are not available, it is appropriate to assess the available evidence, even if it is grade 2 or 3. Indeed, it seems counterintuitive to ignore high quality evidence collected from thousands of patients purely on the grounds that they did not form part of a randomised controlled trial.
In 2000 the Clinical Effectiveness Unit of the Royal College of Surgeons of England conducted a National Comparative Audit of the Surgery for Nasal Polyposis and Chronic Rhinosinusitis covering the work of 298 consultants working in 87 hospital sites in England and Wales (1757). Patients undergoing sinus surgery were prospectively enrolled and followed up in this observational study at 3, 12 and 36 months post-operatively using the SNOT-22 as the main outcome measure. Two thirds (2176) of the 3128 patients participating in this study had CRS with nasal polyps. CRS patients with nasal polyps suffered more frequently from concomitant asthma and ASA-intolerance, had more previous sinonasal surgery, their mean CT score was higher and their mean SNOT-22 symptom score was slightly lower than that of CRS patients without polyps. All forms of sinus surgery were included though the majority were performed endoscopically. Overall there was a high level of satisfaction with the surgery and clinically significant improvement in the SNOT-22 scores was demonstrated at 3, 12 and 36 months (1757) (Figure 6.4.1.). Patients with chronic rhinosinusitis without polyps benefited less from surgery than polyp patients; surgery was indicated in 3.6% of patients at 12 months and 11.8% at 36 months. Major complications were very uncommon. Five year follow up results from almost half of the patients of this audit were published in 2009 (1758): Nineteen percent of patients surveyed eventually underwent revision surgery during these five years, including 15% of patients with CRS without NP. The mean SNOT-22 score for all patients was 28.2, very similar to the results observed at 36 months (27.7), and represents a consistent 14-point improvement over the baseline score. Scores were better for polyp patients (mean = 26.2) than patients with CRS alone (mean = 33.3). (Evidence level IIc)
Long term revision rates in patients with CRSsNP have been shown to be above 10% (and as high as 15-20%)
6.4.4.3. Symptom-specific outcomes
A recent review by Chester et al (1759), screened 289 studies including only studies with 20 or more patients who used symptom severity scores to analyse at least 3 CRS symptoms (facial pressure, nasal obstruction, postnasal discharge, hyposmia and headache). They eventually included 21 studies and 2070 patients with CRS with or without NP a mean of 13 months after ESS: All symptoms improved compared with their preoperative scores by an overall Effect Size of 1.19 (95% confidence interval, 0.96 to 1.41). Nasal obstruction (Effect Size, 1.73) improved the most, with facial pain (ES, 1.13) and postnasal discharge (ES, 1.19) demonstrating moderate improvements. Hyposmia (ES, 0.97) and headache (ES, 0.98) improved the least (Evidence level IV)
RCTs in surgery are notoriously difficult to organise and (as in the case of truly blind "sham" studies), potentially unethical. If these are not available, it is appropriate to assess the available evidence, even if it is grade 2 or 3. Indeed, it seems counterintuitive to ignore high quality evidence collected from thousands of patients purely on the grounds that they did not form part of a randomised controlled trial.
In 2000 the Clinical Effectiveness Unit of the Royal College of Surgeons of England conducted a National Comparative Audit of the Surgery for Nasal Polyposis and Chronic Rhinosinusitis covering the work of 298 consultants working in 87 hospital sites in England and Wales (1757). Patients undergoing sinus surgery were prospectively enrolled and followed up in this observational study at 3, 12 and 36 months post-operatively using the SNOT-22 as the main outcome measure. Two thirds (2176) of the 3128 patients participating in this study had CRS with nasal polyps. CRS patients with nasal polyps suffered more frequently from concomitant asthma and ASA-intolerance, had more previous sinonasal surgery, their mean CT score was higher and their mean SNOT-22 symptom score was slightly lower than that of CRS patients without polyps. All forms of sinus surgery were included though the majority were performed endoscopically. Overall there was a high level of satisfaction with the surgery and clinically significant improvement in the SNOT-22 scores was demonstrated at 3, 12 and 36 months (1757) (Figure 6.4.1.). Patients with chronic rhinosinusitis without polyps benefited less from surgery than polyp patients; surgery was indicated in 3.6% of patients at 12 months and 11.8% at 36 months. Major complications were very uncommon. Five year follow up results from almost half of the patients of this audit were published in 2009 (1758): Nineteen percent of patients surveyed eventually underwent revision surgery during these five years, including 15% of patients with CRS without NP. The mean SNOT-22 score for all patients was 28.2, very similar to the results observed at 36 months (27.7), and represents a consistent 14-point improvement over the baseline score. Scores were better for polyp patients (mean = 26.2) than patients with CRS alone (mean = 33.3). (Evidence level IIc)
Long term revision rates in patients with CRSsNP have been shown to be above 10% (and as high as 15-20%)
6.4.4.3. Symptom-specific outcomes
A recent review by Chester et al (1759), screened 289 studies including only studies with 20 or more patients who used symptom severity scores to analyse at least 3 CRS symptoms (facial pressure, nasal obstruction, postnasal discharge, hyposmia and headache). They eventually included 21 studies and 2070 patients with CRS with or without NP a mean of 13 months after ESS: All symptoms improved compared with their preoperative scores by an overall Effect Size of 1.19 (95% confidence interval, 0.96 to 1.41). Nasal obstruction (Effect Size, 1.73) improved the most, with facial pain (ES, 1.13) and postnasal discharge (ES, 1.19) demonstrating moderate improvements. Hyposmia (ES, 0.97) and headache (ES, 0.98) improved the least (Evidence level IV)
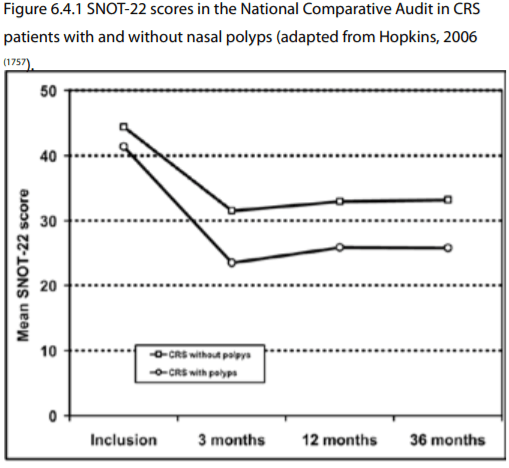
6.4.4.4. Quality of life outcomes
6.4.4.4.1. Generic QOL
A number of studies have shown improvement in generic QOL outcome measures after surgery: Among others, a study published in 2010 (1760) included three hundred thirty-six patients assessed with Short Form 36 Health Survey (SF-36) four months after surgery: the SF36 scores that were significantly decreased before surgery improved and came very close to normal levels. Another study that used the SF-36 in 150 patients after a mean follow-up of 3 years showed statistically significant improvements in QOL scores postoperatively. Importantly, the scores improved to the point of reaching general population levels.
6.4.4.4.2. Disease specific QOL
A variety of disease – specific instruments have been used to assess QOL changes after ESS: These included the Chronic Sinusitis Survey and the Rhinosinusitis Disability Index (1760, 1761), SNOT 20 (1757, 1762), SNOT 22 (1758) RSOM 31 (1763). In all of these studies, evidence of improvement of generic and disease specific QOL with surgery was shown. Deal and Kountakis (1762) using SNOT 20 showed that patients with nasal polyps have worse nasal QOL compared to CRS without NP patients while the English National Comparative Audit using SNOT 22 showed the opposite. However, both studies confirmed that the improvement in QOL after surgery was more pronounced in patients with nasal polyps compared to CRS patients without polyps (1758) (Evidence level IIc).
6.4.4.5. Conclusion
It is fair to say that trials providing high level evidence of the efficacy of ESS for CRS are missing, as only a small percentage of the studies are RCTs, and those who are have inconsistent inclusion criteria, outcome measures and types of interventions making generalisations difficult. Additionally, intervention bias (the variability in surgical techniques and in experience between different surgeons) should not be underestimated. Having said that, there is a significant amount of well designed level II-level III evidence collected from tens of thousands of patients, that endoscopic sinus surgery is safe and is associated with improvements in symptoms scores (especially nasal obstruction and discharge), disease specific and generic QOL as well as objective measures.
6.4.4.4.1. Generic QOL
A number of studies have shown improvement in generic QOL outcome measures after surgery: Among others, a study published in 2010 (1760) included three hundred thirty-six patients assessed with Short Form 36 Health Survey (SF-36) four months after surgery: the SF36 scores that were significantly decreased before surgery improved and came very close to normal levels. Another study that used the SF-36 in 150 patients after a mean follow-up of 3 years showed statistically significant improvements in QOL scores postoperatively. Importantly, the scores improved to the point of reaching general population levels.
6.4.4.4.2. Disease specific QOL
A variety of disease – specific instruments have been used to assess QOL changes after ESS: These included the Chronic Sinusitis Survey and the Rhinosinusitis Disability Index (1760, 1761), SNOT 20 (1757, 1762), SNOT 22 (1758) RSOM 31 (1763). In all of these studies, evidence of improvement of generic and disease specific QOL with surgery was shown. Deal and Kountakis (1762) using SNOT 20 showed that patients with nasal polyps have worse nasal QOL compared to CRS without NP patients while the English National Comparative Audit using SNOT 22 showed the opposite. However, both studies confirmed that the improvement in QOL after surgery was more pronounced in patients with nasal polyps compared to CRS patients without polyps (1758) (Evidence level IIc).
6.4.4.5. Conclusion
It is fair to say that trials providing high level evidence of the efficacy of ESS for CRS are missing, as only a small percentage of the studies are RCTs, and those who are have inconsistent inclusion criteria, outcome measures and types of interventions making generalisations difficult. Additionally, intervention bias (the variability in surgical techniques and in experience between different surgeons) should not be underestimated. Having said that, there is a significant amount of well designed level II-level III evidence collected from tens of thousands of patients, that endoscopic sinus surgery is safe and is associated with improvements in symptoms scores (especially nasal obstruction and discharge), disease specific and generic QOL as well as objective measures.
6.4.5. Functional endoscopic sinus surgery versus
conventional surgery
There have been no studies comparing open ethmoidectomy/ sphenoethmoidectomy with endoscopic sinus surgery for CRS. Lund (1764) examined retrospectively long term outcomes of inferior and middle meatal antrostomy and showed better outcomes in the endoscopic middle meatal antrostomy group. Penttila compared in a RCT ESS and Caldwell Luc and showed marked improvement in 50.7% of the C-L group and in 76.7% of the FESS group at one year (1765). Venkatachalam compared in a RCT conventional surgery with ESS and found that ESS was associated with greater rates of complete relief of symptoms (76% versus 60%) and better overall outcomes (2064).
Conclusion
Functional endoscopic surgery is superior to conventional procedures including polypectomy, Caldwell-Luc, inferior meatal antrostomy and antral irrigations, but superiority to conventional sphenoethmoidectomy is not yet proven.
There have been no studies comparing open ethmoidectomy/ sphenoethmoidectomy with endoscopic sinus surgery for CRS. Lund (1764) examined retrospectively long term outcomes of inferior and middle meatal antrostomy and showed better outcomes in the endoscopic middle meatal antrostomy group. Penttila compared in a RCT ESS and Caldwell Luc and showed marked improvement in 50.7% of the C-L group and in 76.7% of the FESS group at one year (1765). Venkatachalam compared in a RCT conventional surgery with ESS and found that ESS was associated with greater rates of complete relief of symptoms (76% versus 60%) and better overall outcomes (2064).
Conclusion
Functional endoscopic surgery is superior to conventional procedures including polypectomy, Caldwell-Luc, inferior meatal antrostomy and antral irrigations, but superiority to conventional sphenoethmoidectomy is not yet proven.
6.4.6. ESS modifications / extent of surgery
While there is some evidence that more extensive surgery (larger middle meatal antrostomy, widening of the frontal recess, more extensive sphenoithmoidectomy) may be associated with better objective outcomes, it is suggested to tailor the extent of surgery to the extent of disease.
Extent of surgery may vary from mere uncinectomy to radical sphenoethmoidectomy with middle turbinate resection. In several studies, the extent of sinus surgery on various outcome parameters was investigated in CRS patients, not differentiating between CRS with and without polyps. In a prospective trial, 65 CRS patients with and without polyps were randomized to undergo limited endonasal functional surgery (uncinectomy) and a more extensive functional procedure including sphenoethmoidectomy and wide opening of the frontal recess. Disease extent was similar in both treatment arms. Outcome parameters included symptom scores, rhinoscopy scores and nasal saccharin transport time (1766). Outcome parameters revealed no relevant differences after 3, 6 and 12 months, however, recall rates lower than 60% limit the usefulness of this study . Jankowski and co-authors retrospectively compared a case series of 37 CRS patients with extensive nasal polyps treated with FESS with a historical group of 36 patients with similar disease extent treated with radical sphenoethmoidectomy and middle turbinate resection (1767). Outcome parameters assessed 5 years following surgery included a mailed questionnaire on nasal symptoms, the number of patients with revision surgery, and nasal endoscopy scores at a follow up visit. Recall was below 80% and differed significantly between the two groups. The radical surgical procedure yielded better symptom scores, less recurrences, and better endoscopic scores at the follow up visit (Evidence level IV).
In a randomized trial, 1,106 matched CRS patients with and without polyps, who underwent similar functional endonasal sinus surgery with (509 patients) or without (597 patients) partial middle turbinate resection (1768). Partial middle turbinate resection was associated with less adhesion formation and less revision. Complications specific to partial middle turbinate resection were not observed (Evidence level Ib). In a study by Marchioni et al, 22 patients with middle turbinate resection and 34 patients with turbinate preservation were followed for three years. Patients without middle turbinate resection were shown to have earlier relapse of polyposis as judged by endoscopy examination (1769). A recent non randomised prospective study compared patients on which 2/3 of the medial turbinate were removed (on medical reasons) with those where it was preserved (1770). It is interesting to note that patients with MT resection were more likely to have asthma , aspirin intolerance, nasal polyposis, and prior sinus surgery, and higher baseline disease burden. Although there were no differences in generic or disease specific QOL measures, patients undergoing MT resection were more likely to show improvements in mean endoscopy and olfaction.
The patency rate after large middle meatal antrostomy and undisturbed maxillary ostium in endoscopic sinus surgery for CRS without nasal polyps was compared in a recent (2011) study: thirty patients with CRS without NP underwent randomized endoscopic sinus surgery (1771). A large middle meatal antrostomy was performed on one side, whereas on the other side an uncinectomy preserving the natural maxillary ostium was done. The patency rates of the middle meatal antrostomy were significantly higher and the radiological Lund-McKay score was lower 9 months after surgery when compared to the side with the undisturbed maxillary ostium. This difference however did not translate to improved subjective outcomes (Evidence level Ib).
While there is some evidence that more extensive surgery (larger middle meatal antrostomy, widening of the frontal recess, more extensive sphenoithmoidectomy) may be associated with better objective outcomes, it is suggested to tailor the extent of surgery to the extent of disease.
Extent of surgery may vary from mere uncinectomy to radical sphenoethmoidectomy with middle turbinate resection. In several studies, the extent of sinus surgery on various outcome parameters was investigated in CRS patients, not differentiating between CRS with and without polyps. In a prospective trial, 65 CRS patients with and without polyps were randomized to undergo limited endonasal functional surgery (uncinectomy) and a more extensive functional procedure including sphenoethmoidectomy and wide opening of the frontal recess. Disease extent was similar in both treatment arms. Outcome parameters included symptom scores, rhinoscopy scores and nasal saccharin transport time (1766). Outcome parameters revealed no relevant differences after 3, 6 and 12 months, however, recall rates lower than 60% limit the usefulness of this study . Jankowski and co-authors retrospectively compared a case series of 37 CRS patients with extensive nasal polyps treated with FESS with a historical group of 36 patients with similar disease extent treated with radical sphenoethmoidectomy and middle turbinate resection (1767). Outcome parameters assessed 5 years following surgery included a mailed questionnaire on nasal symptoms, the number of patients with revision surgery, and nasal endoscopy scores at a follow up visit. Recall was below 80% and differed significantly between the two groups. The radical surgical procedure yielded better symptom scores, less recurrences, and better endoscopic scores at the follow up visit (Evidence level IV).
In a randomized trial, 1,106 matched CRS patients with and without polyps, who underwent similar functional endonasal sinus surgery with (509 patients) or without (597 patients) partial middle turbinate resection (1768). Partial middle turbinate resection was associated with less adhesion formation and less revision. Complications specific to partial middle turbinate resection were not observed (Evidence level Ib). In a study by Marchioni et al, 22 patients with middle turbinate resection and 34 patients with turbinate preservation were followed for three years. Patients without middle turbinate resection were shown to have earlier relapse of polyposis as judged by endoscopy examination (1769). A recent non randomised prospective study compared patients on which 2/3 of the medial turbinate were removed (on medical reasons) with those where it was preserved (1770). It is interesting to note that patients with MT resection were more likely to have asthma , aspirin intolerance, nasal polyposis, and prior sinus surgery, and higher baseline disease burden. Although there were no differences in generic or disease specific QOL measures, patients undergoing MT resection were more likely to show improvements in mean endoscopy and olfaction.
The patency rate after large middle meatal antrostomy and undisturbed maxillary ostium in endoscopic sinus surgery for CRS without nasal polyps was compared in a recent (2011) study: thirty patients with CRS without NP underwent randomized endoscopic sinus surgery (1771). A large middle meatal antrostomy was performed on one side, whereas on the other side an uncinectomy preserving the natural maxillary ostium was done. The patency rates of the middle meatal antrostomy were significantly higher and the radiological Lund-McKay score was lower 9 months after surgery when compared to the side with the undisturbed maxillary ostium. This difference however did not translate to improved subjective outcomes (Evidence level Ib).
6.4.6.1. Balloon catheter
In a recent Cochrane review (2065) as well as in an evidence – based review published in 2011 (1772), Batra et al. assessed the evidence available for new ballon catheter systems for sinus surgery in CRS: There have not been any prospective comparative studies comparing it to standard FESS techniques. The one retrospective comparative study referred to patients with recurrent acute or mild CRS and the patients elected themselves the way of treatments, while follow up was 3 months, precluding any meaningful conclusions. On the other hand, a number of prospective multicentre studies assessing the balloon catheter systems have been published, which confirm a good safety profile (albeit not complication – free (1773)), but have unclear inclusion criteria, making their results difficult to generalise. Overall, the place of these systems in the sinus surgeon's armamentarium remains unclear (Evidence level IV).
Conclusion
Although not fully evidence based, the extent of surgery is frequently tailored to the extent of disease, which may appear as a reasonable approach. In primary paranasal sinus surgery, surgical conservatism is recommended. The decision to preserve or resect the middle turbinate can be left to the discretion of the surgeon based on its disease status. There is not enough data to support the use of balloon catheters as an alternative to standard endoscopic sinus surgery techniques.
In a recent Cochrane review (2065) as well as in an evidence – based review published in 2011 (1772), Batra et al. assessed the evidence available for new ballon catheter systems for sinus surgery in CRS: There have not been any prospective comparative studies comparing it to standard FESS techniques. The one retrospective comparative study referred to patients with recurrent acute or mild CRS and the patients elected themselves the way of treatments, while follow up was 3 months, precluding any meaningful conclusions. On the other hand, a number of prospective multicentre studies assessing the balloon catheter systems have been published, which confirm a good safety profile (albeit not complication – free (1773)), but have unclear inclusion criteria, making their results difficult to generalise. Overall, the place of these systems in the sinus surgeon's armamentarium remains unclear (Evidence level IV).
Conclusion
Although not fully evidence based, the extent of surgery is frequently tailored to the extent of disease, which may appear as a reasonable approach. In primary paranasal sinus surgery, surgical conservatism is recommended. The decision to preserve or resect the middle turbinate can be left to the discretion of the surgeon based on its disease status. There is not enough data to support the use of balloon catheters as an alternative to standard endoscopic sinus surgery techniques.
6.4.8. Revision sinus surgery
Approximately 20% of operated patients respond unsatisfactorily to sinus surgery with concomitant medical therapy and eventually require a secondary surgical procedure (1758). Middle turbinate lateralisation, adhesions and scar formation in the middle meatus, an incompletely resected uncinate process, and retained ethmoid cells are frequent findings in patients undergoing revision surgery (1778). Previous revision surgery, extensive polyps, bronchial asthma, ASAintolerance and cystic fibrosis are predictors of revision surgery (1762, 1775, 1779). Inflammatory involvement of underlying bone may also be of significance (1388). Technical issues of sinus revision surgery have be reported by Cohen and Kennedy (1780) and Javer more recently (1781). A more extensive surgical procedure and also external approaches may be indicated (1767, 1782). Success rates of revision endoscopic sinus surgery have been reported to range between 50 and 70% (762, 1783). Complication rates of revision surgery are higher when compared with initial surgery and approximate 1%, but may be as high as 7% (1784, 1785), McMains and Kountakis also reported the results of 59 CRS patients with nasal polyps after revision surgery (1779). Consistent with the results of the National Comparative Audit (1757) and the comparative study by Deal and co-workers (1762), CRS patients without polyps had higher SNOT scores preoperatively (more severe symptoms), less previous surgeries, and a lower CT score preoperatively than CRS patients with polyps. However, the improvement of outcome parameters after revision surgery was significant and comparable with the improvement in CRS patients without polyps, greater even after 5 years (1758) in the case of the English National Audit study. The same was found in, a recent comparative study (1786) that, using Rhinosinusitis Disability Index (RSDI) and Chronic Sinusitis Survey (CSS) showed that the improvement in QOL is the same in patients undergoing revision or primary surgery, although the endoscopic improvement was CRS without NP patients undergoing revision surgery. Schlosser (1787) and Ferguson (1788) looked at patients who underwent multiple failed procedures: such patients often harbour subtle humoral immunodeficiencies, systemic granulomatous or eosinophilic syndromes. There are a handful of observational studies suggesting that patients with aspirin exacerbated respiratory disease may benefit from aspirin desensitization, while dealing with allergy with antihistamines and desensitisation, as well as long term, culture driven antibiotics and an intensive program of nasal lavage may improve outcome.
Conclusion
Revision endonasal sinus surgery is only indicated, if medical treatment is not sufficiently effective. Substantial symptomatic improvement is generally observed in both, CRS with and without polyps, though the improvement maybe somewhat less than after primary surgery. Complication rates and particularly the risk of disease recurrence are higher than after primary surgery. Some patients still suffer from CRS symptoms after several extensive surgical procedures. CT scans frequently show mucosal alterations adjacent to osteitic bony margins in an extensively operated sinus system. As a rule, revision surgery is not indicated in these patients but radical surgery can be an option (1782).
Approximately 20% of operated patients respond unsatisfactorily to sinus surgery with concomitant medical therapy and eventually require a secondary surgical procedure (1758). Middle turbinate lateralisation, adhesions and scar formation in the middle meatus, an incompletely resected uncinate process, and retained ethmoid cells are frequent findings in patients undergoing revision surgery (1778). Previous revision surgery, extensive polyps, bronchial asthma, ASAintolerance and cystic fibrosis are predictors of revision surgery (1762, 1775, 1779). Inflammatory involvement of underlying bone may also be of significance (1388). Technical issues of sinus revision surgery have be reported by Cohen and Kennedy (1780) and Javer more recently (1781). A more extensive surgical procedure and also external approaches may be indicated (1767, 1782). Success rates of revision endoscopic sinus surgery have been reported to range between 50 and 70% (762, 1783). Complication rates of revision surgery are higher when compared with initial surgery and approximate 1%, but may be as high as 7% (1784, 1785), McMains and Kountakis also reported the results of 59 CRS patients with nasal polyps after revision surgery (1779). Consistent with the results of the National Comparative Audit (1757) and the comparative study by Deal and co-workers (1762), CRS patients without polyps had higher SNOT scores preoperatively (more severe symptoms), less previous surgeries, and a lower CT score preoperatively than CRS patients with polyps. However, the improvement of outcome parameters after revision surgery was significant and comparable with the improvement in CRS patients without polyps, greater even after 5 years (1758) in the case of the English National Audit study. The same was found in, a recent comparative study (1786) that, using Rhinosinusitis Disability Index (RSDI) and Chronic Sinusitis Survey (CSS) showed that the improvement in QOL is the same in patients undergoing revision or primary surgery, although the endoscopic improvement was CRS without NP patients undergoing revision surgery. Schlosser (1787) and Ferguson (1788) looked at patients who underwent multiple failed procedures: such patients often harbour subtle humoral immunodeficiencies, systemic granulomatous or eosinophilic syndromes. There are a handful of observational studies suggesting that patients with aspirin exacerbated respiratory disease may benefit from aspirin desensitization, while dealing with allergy with antihistamines and desensitisation, as well as long term, culture driven antibiotics and an intensive program of nasal lavage may improve outcome.
Conclusion
Revision endonasal sinus surgery is only indicated, if medical treatment is not sufficiently effective. Substantial symptomatic improvement is generally observed in both, CRS with and without polyps, though the improvement maybe somewhat less than after primary surgery. Complication rates and particularly the risk of disease recurrence are higher than after primary surgery. Some patients still suffer from CRS symptoms after several extensive surgical procedures. CT scans frequently show mucosal alterations adjacent to osteitic bony margins in an extensively operated sinus system. As a rule, revision surgery is not indicated in these patients but radical surgery can be an option (1782).
6.5. Treatment with corticosteroids in CRSwNP
6.5.1. Introduction
In this chapter a differentiation is made between CRSsNP and CRSwNP. Readers have to realize that often in studies no clear difference is made between these two patients groups. Sometimes for this reason studies are discussed in both the parts on CRSsNP as the parts of CRSwNP. In studies on the treatment of CRSwNP, it is of value to look separately at the effect on rhinitis symptoms associated with polyposis and the effect on the size of nasal polyps per se. There are many symptom aspects of CRSwNP and we have also included an objective measure of nasal obstruction, nasal peak inspiratory flow (PNIF), as this was the most commonly reported objective measure behind endoscopy.
6.5.2. Local corticosteroid (INCS) in chronic rhinosinusitis with nasal polyps
Considering the number of studies in the literature, only RCTs will be referred to in this summary. INCS for CRSwNP encompasses range of different treatment regimes. These have been carefully described in the Table of study characteristics (Table 6.5.1.).
6.5.2.1. Inclusion criteria and exclusion criteria
Inclusion criteria
Patients with benign nasal polyps diagnosed clinically with either:
Topical steroids versus no intervention
Topical steroids versus placebo
Topical and oral steroids versus oral steroids only
6.5.2.3. Flow chart
A total of 873 references were retrieved: three more records were identified from references of retrieved studies. 735 of these were removed in first-level screening (i.e. removal of duplicates and clearly irrelevant references), leaving 141 references for further consideration. Title and abstracts were screened and 93 studies were subsequently removed. Fortyeight full texts were assessed for eligibility. Three papers were abstracts of presentations at academic meetings of included studies. One paper pooled data from two included studies for reanalysis. Three non-randomized studies and neither two studies comparing topical steroid to neither placebo nor no intervention were excluded. Thirty-nine studies were included. A flow chart of study retrieval and selection is provided in Figure 6.5.1.
6.5.2.4. Included studies
There were 3,532 participants totally in 38 included studies. The mean age of patients was 48.2 years. The percentage of men was 66.6. The characteristics of included studies are listed as Table 6.5.1.
6.5.2.5. Summary of data
Thirtyfour trials (92%) compared topical steroid against placebo (Aukema 2005; Bross-Soriano 2004; Chalton 1985; Dingsor 1985; Djikstra 2004; Drettner 1982; Ehnhage 2009; Filiaci 2000; Hartwig 1988; Holmberg 1997; Holmstrom 1999; Holopainen 1982; Jankowski 2001; Jankowski 2009; Johansen 1993; Johansson 2002; Jorissen 2009; Keith 2000; Lang 1983; Lildholdt 1995; Lund 1998; Mastalerz 1997; Mygind 1975; Olsson 2010; Passali 2003; Penttila 2000; Rowe-Jones 2005; Ruhno1990; Small 2005; Stjarne 2006; Stjarne 2006b; Stjarne 2009; Tos 1998; Vlckova 2009) (1109, 1172, 1426, 1668, 1674, 1789-1816). Among these, eight trials also compared low dose to high dose of topical steroid (Djikstra 2004; Filiaci 2000; Jankowski 2001; Lildholdt 1995; Penttila 2000; Small 2005; Stjarne 2006; Tos 1998) (1668, 1794, 1804, 1808, 1810, 1813, 1815-1817) and three trials also compared two steroid agents, fluticasone propionate and beclomethasone dipropionate (Bross-Soriano 2004; Holmberg 1997; Lund 1998) (1109, 1790, 1796). Three trials (8%) compared topical steroid against no intervention (El Naggar 1995; Jurkiewicz 2004; Karlsson 1982) (1818-1820).
Twenty (55%) included studies were fully or partially sponsored by pharmaceutical companies; Glaxo (Aukema 2005; Djikstra 2004; Ehnhage 2009; Holmberg 1997; Keith 2000; Lund 1998; Mastalerz 1997; Mygind 1975; Olsson 2010; Penttila 2000; Rowe-Jones 2005) (1109, 1172, 1426, 1668, 1789, 1796, 1802, 1805, 1806, 1808, 1821). Astra (Johansen 1993; Johansson 2002; Ruhno1990; Tos 1998) (1800, 1801, 1809, 1813) and Schering Plough (Jorissen 2009; Small 2005; Stjarne 2006; Stjarne 2006b; Stjarne 2009) (1674, 1810-1812, 1816).
The steroid agents used were differed across the studies:
6.5.2.6. Meta-analysis
When compared to placebo, pooled data analyses of symptoms, polyp size, polyp recurrence and nasal airflow demonstrated significant benefit in the topical steroid group. Although these outcomes were reported in various ways across studies such as the final value, the change of value after intervention and the proportion of responders, all meta-analyses show the same results favouring topical steroid. Although 32, 29 and 22 studies reported symptoms, polyp size and nasal airflow, data from only 9, 13 and 9 studies respectively can be pooled for meta-analysis. Most studies do not provide numeric data of the outcomes or do not show any of standard deviation, standard error, 95%CI, range nor interquartile range. Data from only one study was analyzed for change in CT scan (1789), and quality of life (1172). No difference from placebo was found in these 2 outcomes. Olfactory outcomes are mentioned in 22 studies (1426, 1797, 1800, 1802, 1804, 1808, 1810-1814, 1816, 1818) and with mixed benefit to INCS. More studies may be helpful to make conclusions for these three outcomes.
6.5.2.6.1. Symptom improvement (score or responders)
Data addressing the change in combined symptom scores was available from seven studies (1674, 1794, 1798, 1801, 1805, 1806, 1814) and could be combined in the meta-analysis. The pooled results significantly favoured the topical steroid group (SMD -0.46; 95% CI -0.65 to -0.27), p<0.00001; seven trials, 445 patients) (Figure 6.5.2.A). Data addressing the proportion of responders in symptoms was available from four studies (1794, 1796, 1806, 1808). The pooled results significantly favoured the topical steroid group (RR (Non-event) 0.59; 95% CI 0.46 to 0.78), p=0.0001 (Figure 6.5.2.B).
6.5.2.6.2. Polyp size (score, change or responders on endoscopy)
Data addressing the final value of polyp score at the endpoint was available from three studies (Dingsor 1985; Hartwig 1988; Johansson 2002) (1792, 1795, 1801) and could be combined in the meta-analysis. The pooled results significantly favoured the topical steroid group (SMD -0.49; 95% CI -0.77 to -0.21), p=0.0007 (Figure 6.5.3.A). Data addressing the change in polyp score was available from three studies (1806, 1814, 1815). and could be combined in the meta-analysis. The pooled results significantly favoured the topical steroid group (SMD -0.73; 95% CI -1.00 to -0.46), p<0.00001 (Figure 6.5.3.B). Data addressing the proportion of responders in polyp size was available from eight studies (1791, 1797, 1798, 1802, 1803, 1808, 1811, 1814) and could be combined in the meta-analysis. The pooled results significantly favoured the topical steroid group (RR (Non-event) 0.74; 95% CI 0.67 to 0.81), p<0.00001. (Figure 6.5.3.C)
6.5.2.6.3. Nasal breathing (score, change or responders on Peak Nasal Inspiratory Flow (PNIF))
Data addressing the peak nasal inspiratory flow was available from seven studies (1789, 1798, 1799, 1801, 1805, 1809, 1814) and could be combined in the meta-analysis. The pooled results significantly favoured the topical steroid group (MD 22.04; 95% CI 13.29 to 30.80), p<0.00001 (Figure 6.5.4a). Data addressing the change in nasal airflow was available from three studies (Ehnhage 2009; Holmstrom 1999; Ruhno1990) (1797, 1809, 1818) and could be combined in the meta-analysis. The pooled results significantly favoured the topical steroid group (SMD -0.57; 95% CI -0.85 to -0.29), p=0.0001 (Figure 6.5.4b). Data addressing the proportion of responders in nasal airflow was available from two studies (Chalton 1985; Ruhno1990) (1791, 1809) which significantly favoured the topical steroid group (RR (Non-event) 0.55; 95% CI 0.33 to 0.89), p=0.02 (Figure 6.5.4.c.).
The standardised mean difference (SMD) and 95% CIs for continuous data such as post-intervention scores or change in symptom scores. The risk ratio (RR) and 95% CI of responsiveness was used at a specific time point for dichotomous data such as number of patients responding to treatment. The intervention effects were pooled when trials were sufficiently homogeneous. The SDs were imputed from p values for Lund 1998 after assuming parametric data. A fixed-effect model was used and assumed that each study was estimating the same quantity.
6.5.2.7. Subgroup analysis
Subgroup analysis was performed as follows.
The 38 included studies were diverse, both clinically and methodologically. Variability included sinus surgery status, topical delivery methods, polyp severity, steroid agent used and regimes. Subgroup analyses were performed to investigate heterogeneity.
6.5.1. Introduction
In this chapter a differentiation is made between CRSsNP and CRSwNP. Readers have to realize that often in studies no clear difference is made between these two patients groups. Sometimes for this reason studies are discussed in both the parts on CRSsNP as the parts of CRSwNP. In studies on the treatment of CRSwNP, it is of value to look separately at the effect on rhinitis symptoms associated with polyposis and the effect on the size of nasal polyps per se. There are many symptom aspects of CRSwNP and we have also included an objective measure of nasal obstruction, nasal peak inspiratory flow (PNIF), as this was the most commonly reported objective measure behind endoscopy.
6.5.2. Local corticosteroid (INCS) in chronic rhinosinusitis with nasal polyps
Considering the number of studies in the literature, only RCTs will be referred to in this summary. INCS for CRSwNP encompasses range of different treatment regimes. These have been carefully described in the Table of study characteristics (Table 6.5.1.).
6.5.2.1. Inclusion criteria and exclusion criteria
Inclusion criteria
Patients with benign nasal polyps diagnosed clinically with either:
- endoscopic evidence of nasal polyps; or/and
- radiological evidence of nasal polyps
- Antrochoanal polyps (benign polyps originating from the mucosa of the maxillary sinus)
- Malignant polyps
- Cystic fibrosis
- Primary ciliary dyskinesia
Topical steroids versus no intervention
Topical steroids versus placebo
Topical and oral steroids versus oral steroids only
6.5.2.3. Flow chart
A total of 873 references were retrieved: three more records were identified from references of retrieved studies. 735 of these were removed in first-level screening (i.e. removal of duplicates and clearly irrelevant references), leaving 141 references for further consideration. Title and abstracts were screened and 93 studies were subsequently removed. Fortyeight full texts were assessed for eligibility. Three papers were abstracts of presentations at academic meetings of included studies. One paper pooled data from two included studies for reanalysis. Three non-randomized studies and neither two studies comparing topical steroid to neither placebo nor no intervention were excluded. Thirty-nine studies were included. A flow chart of study retrieval and selection is provided in Figure 6.5.1.
6.5.2.4. Included studies
There were 3,532 participants totally in 38 included studies. The mean age of patients was 48.2 years. The percentage of men was 66.6. The characteristics of included studies are listed as Table 6.5.1.
6.5.2.5. Summary of data
Thirtyfour trials (92%) compared topical steroid against placebo (Aukema 2005; Bross-Soriano 2004; Chalton 1985; Dingsor 1985; Djikstra 2004; Drettner 1982; Ehnhage 2009; Filiaci 2000; Hartwig 1988; Holmberg 1997; Holmstrom 1999; Holopainen 1982; Jankowski 2001; Jankowski 2009; Johansen 1993; Johansson 2002; Jorissen 2009; Keith 2000; Lang 1983; Lildholdt 1995; Lund 1998; Mastalerz 1997; Mygind 1975; Olsson 2010; Passali 2003; Penttila 2000; Rowe-Jones 2005; Ruhno1990; Small 2005; Stjarne 2006; Stjarne 2006b; Stjarne 2009; Tos 1998; Vlckova 2009) (1109, 1172, 1426, 1668, 1674, 1789-1816). Among these, eight trials also compared low dose to high dose of topical steroid (Djikstra 2004; Filiaci 2000; Jankowski 2001; Lildholdt 1995; Penttila 2000; Small 2005; Stjarne 2006; Tos 1998) (1668, 1794, 1804, 1808, 1810, 1813, 1815-1817) and three trials also compared two steroid agents, fluticasone propionate and beclomethasone dipropionate (Bross-Soriano 2004; Holmberg 1997; Lund 1998) (1109, 1790, 1796). Three trials (8%) compared topical steroid against no intervention (El Naggar 1995; Jurkiewicz 2004; Karlsson 1982) (1818-1820).
Twenty (55%) included studies were fully or partially sponsored by pharmaceutical companies; Glaxo (Aukema 2005; Djikstra 2004; Ehnhage 2009; Holmberg 1997; Keith 2000; Lund 1998; Mastalerz 1997; Mygind 1975; Olsson 2010; Penttila 2000; Rowe-Jones 2005) (1109, 1172, 1426, 1668, 1789, 1796, 1802, 1805, 1806, 1808, 1821). Astra (Johansen 1993; Johansson 2002; Ruhno1990; Tos 1998) (1800, 1801, 1809, 1813) and Schering Plough (Jorissen 2009; Small 2005; Stjarne 2006; Stjarne 2006b; Stjarne 2009) (1674, 1810-1812, 1816).
The steroid agents used were differed across the studies:
- Fluticasone propionate was studied in 15 trials (Aukema 2005; Bross-Soriano 2004; Djikstra 2004; Ehnhage 2009; Holmberg 1997; Holmstrom 1999; Jankowski 2009; Jurkiewicz 2004; Keith 2000; Lund 1998; Mastalerz 1997; Olsson 2010; Penttila 2000; Rowe-Jones 2005; Vlckova 2009) (1109, 1172, 1426, 1668, 1789, 1790, 1796, 1797, 1799, 1802, 1805, 1808, 1814, 1819, 1821).
- Beclomethasone dipropionate was studied in 7 trials (BrossSoriano 2004; El Naggar 1995; Holmberg 1997; Lund 1998; Karlsson 1982; Lang 1983; Mygind 1975) (1109, 1790, 1796, 1803, 1806, 1818, 1820).
- Betamethasone sodium phospate was studied in 1 trial (Chalton 1985) (1791).
- Mometasone furoate was studied in 6 trials (Jorissen 2009; Passali 2003; Small 2005; Stjarne 2006; Stjarne 2006b; Stjarne 2009) (1674, 1807, 1810-1812, 1816, 1822-1825).
- Flunisolide was studied in 2 trials (Dingsor 1985; Drettner 1982) (1792, 1793).
- Budesonide was studied in 9 trials (Filiaci 2000; Hartwig 1988; Holopainen 1982; Jankowski 2001; Johansen 1993; Johansson 2002; Lildholdt 1995; Ruhno1990; Tos 1998) (1794, 1795, 1798, 1800, 1801, 1804, 1809, 1813, 1815).
6.5.2.6. Meta-analysis
When compared to placebo, pooled data analyses of symptoms, polyp size, polyp recurrence and nasal airflow demonstrated significant benefit in the topical steroid group. Although these outcomes were reported in various ways across studies such as the final value, the change of value after intervention and the proportion of responders, all meta-analyses show the same results favouring topical steroid. Although 32, 29 and 22 studies reported symptoms, polyp size and nasal airflow, data from only 9, 13 and 9 studies respectively can be pooled for meta-analysis. Most studies do not provide numeric data of the outcomes or do not show any of standard deviation, standard error, 95%CI, range nor interquartile range. Data from only one study was analyzed for change in CT scan (1789), and quality of life (1172). No difference from placebo was found in these 2 outcomes. Olfactory outcomes are mentioned in 22 studies (1426, 1797, 1800, 1802, 1804, 1808, 1810-1814, 1816, 1818) and with mixed benefit to INCS. More studies may be helpful to make conclusions for these three outcomes.
6.5.2.6.1. Symptom improvement (score or responders)
Data addressing the change in combined symptom scores was available from seven studies (1674, 1794, 1798, 1801, 1805, 1806, 1814) and could be combined in the meta-analysis. The pooled results significantly favoured the topical steroid group (SMD -0.46; 95% CI -0.65 to -0.27), p<0.00001; seven trials, 445 patients) (Figure 6.5.2.A). Data addressing the proportion of responders in symptoms was available from four studies (1794, 1796, 1806, 1808). The pooled results significantly favoured the topical steroid group (RR (Non-event) 0.59; 95% CI 0.46 to 0.78), p=0.0001 (Figure 6.5.2.B).
6.5.2.6.2. Polyp size (score, change or responders on endoscopy)
Data addressing the final value of polyp score at the endpoint was available from three studies (Dingsor 1985; Hartwig 1988; Johansson 2002) (1792, 1795, 1801) and could be combined in the meta-analysis. The pooled results significantly favoured the topical steroid group (SMD -0.49; 95% CI -0.77 to -0.21), p=0.0007 (Figure 6.5.3.A). Data addressing the change in polyp score was available from three studies (1806, 1814, 1815). and could be combined in the meta-analysis. The pooled results significantly favoured the topical steroid group (SMD -0.73; 95% CI -1.00 to -0.46), p<0.00001 (Figure 6.5.3.B). Data addressing the proportion of responders in polyp size was available from eight studies (1791, 1797, 1798, 1802, 1803, 1808, 1811, 1814) and could be combined in the meta-analysis. The pooled results significantly favoured the topical steroid group (RR (Non-event) 0.74; 95% CI 0.67 to 0.81), p<0.00001. (Figure 6.5.3.C)
6.5.2.6.3. Nasal breathing (score, change or responders on Peak Nasal Inspiratory Flow (PNIF))
Data addressing the peak nasal inspiratory flow was available from seven studies (1789, 1798, 1799, 1801, 1805, 1809, 1814) and could be combined in the meta-analysis. The pooled results significantly favoured the topical steroid group (MD 22.04; 95% CI 13.29 to 30.80), p<0.00001 (Figure 6.5.4a). Data addressing the change in nasal airflow was available from three studies (Ehnhage 2009; Holmstrom 1999; Ruhno1990) (1797, 1809, 1818) and could be combined in the meta-analysis. The pooled results significantly favoured the topical steroid group (SMD -0.57; 95% CI -0.85 to -0.29), p=0.0001 (Figure 6.5.4b). Data addressing the proportion of responders in nasal airflow was available from two studies (Chalton 1985; Ruhno1990) (1791, 1809) which significantly favoured the topical steroid group (RR (Non-event) 0.55; 95% CI 0.33 to 0.89), p=0.02 (Figure 6.5.4.c.).
The standardised mean difference (SMD) and 95% CIs for continuous data such as post-intervention scores or change in symptom scores. The risk ratio (RR) and 95% CI of responsiveness was used at a specific time point for dichotomous data such as number of patients responding to treatment. The intervention effects were pooled when trials were sufficiently homogeneous. The SDs were imputed from p values for Lund 1998 after assuming parametric data. A fixed-effect model was used and assumed that each study was estimating the same quantity.
6.5.2.7. Subgroup analysis
Subgroup analysis was performed as follows.
- Surgical status
- Patients with prior sinus surgery versus those without sinus surgery.
- Topical delivery method
- Nasal drops versus nasal sprays versus sinus (direct cannulation, irrigation post-surgery) delivery method.
- Corticosteroid type
- Modern corticosteroids (mometasone, fluticasone, ciclesonide) versus first-generation corticosteroids (budesonide, beclomethasone, betamethasone, triamcinolone, dexamethasone)
The 38 included studies were diverse, both clinically and methodologically. Variability included sinus surgery status, topical delivery methods, polyp severity, steroid agent used and regimes. Subgroup analyses were performed to investigate heterogeneity.
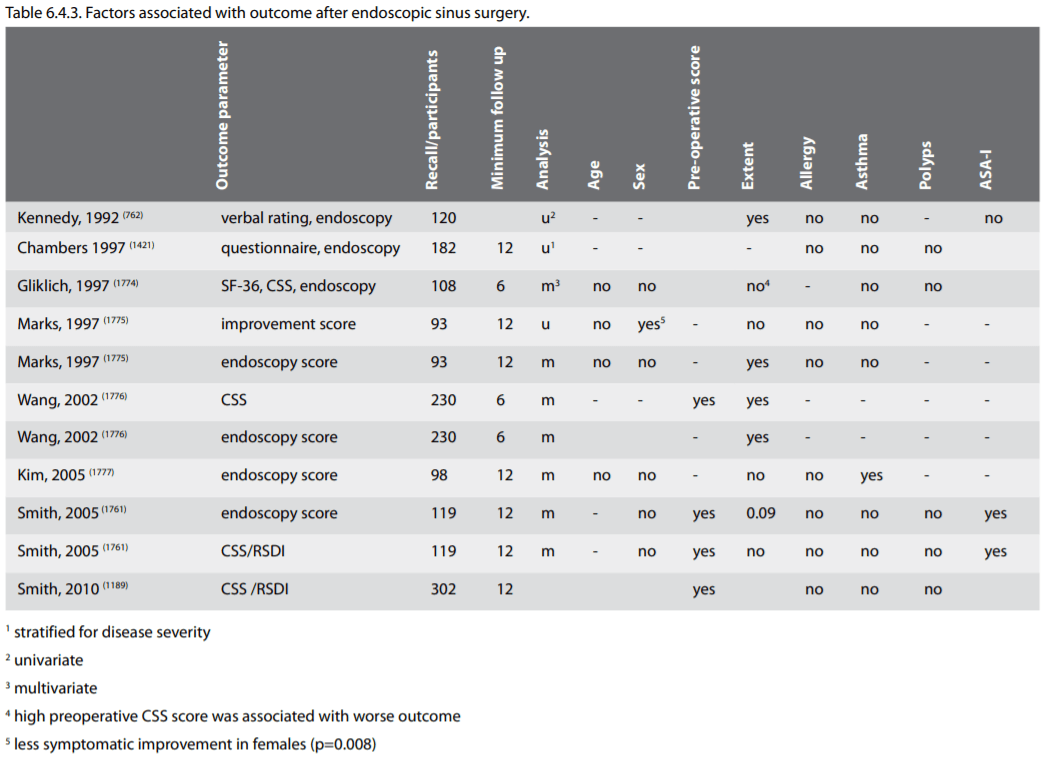


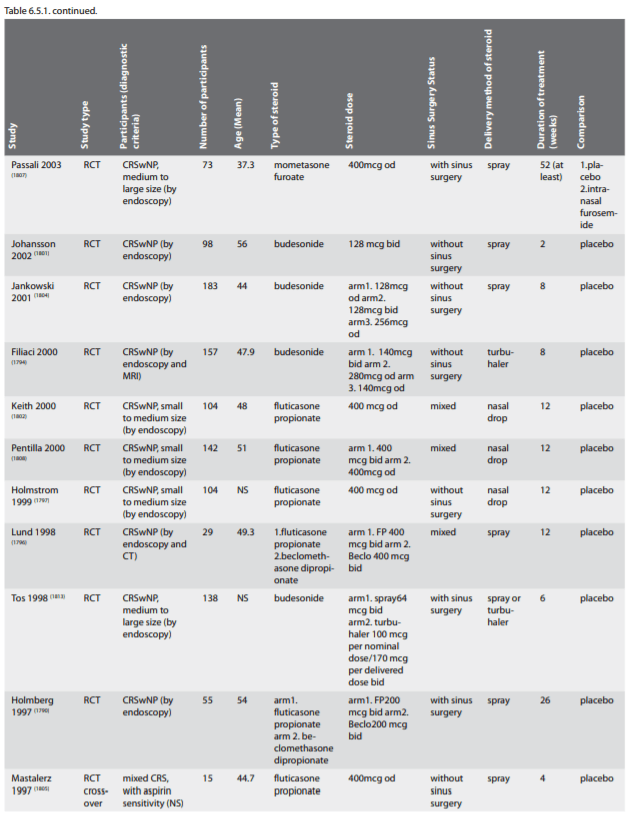
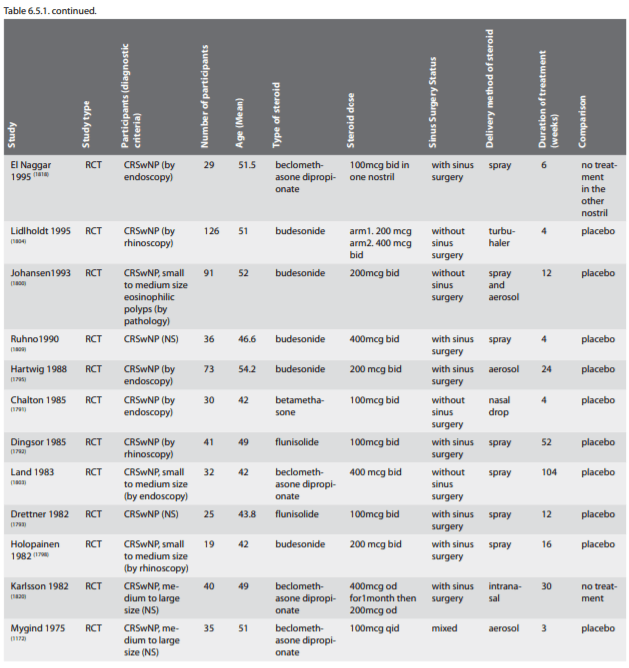

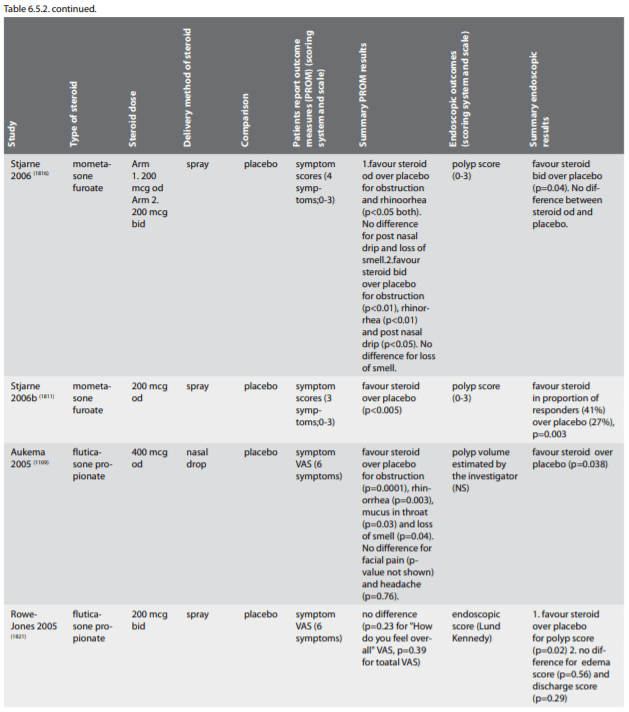
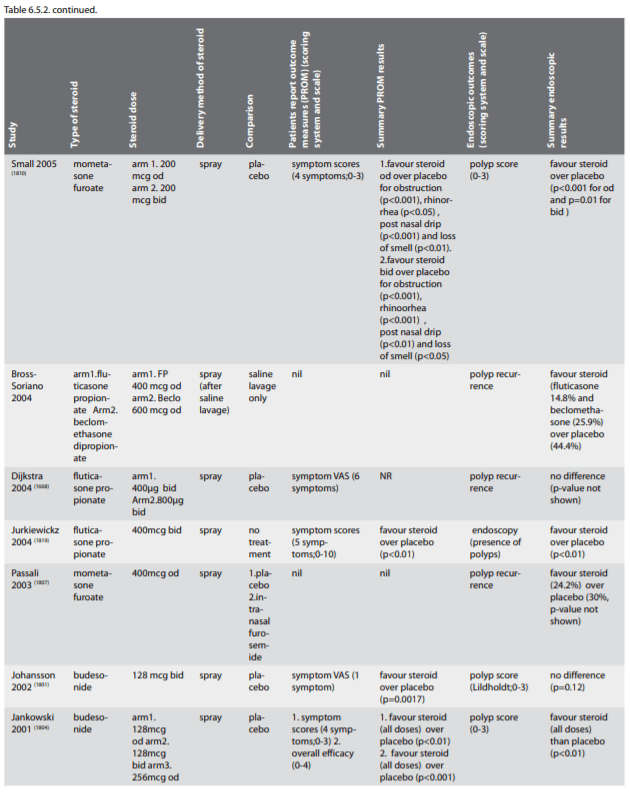
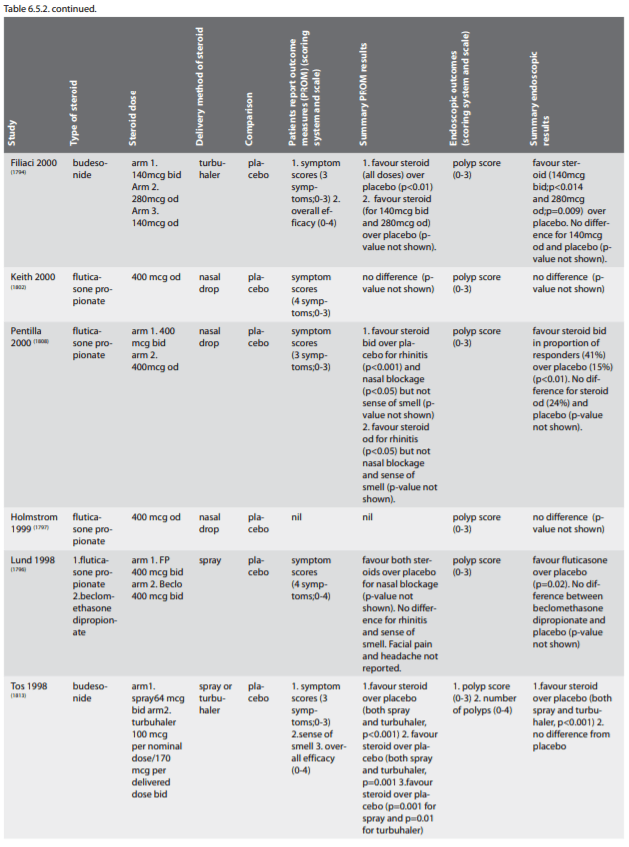
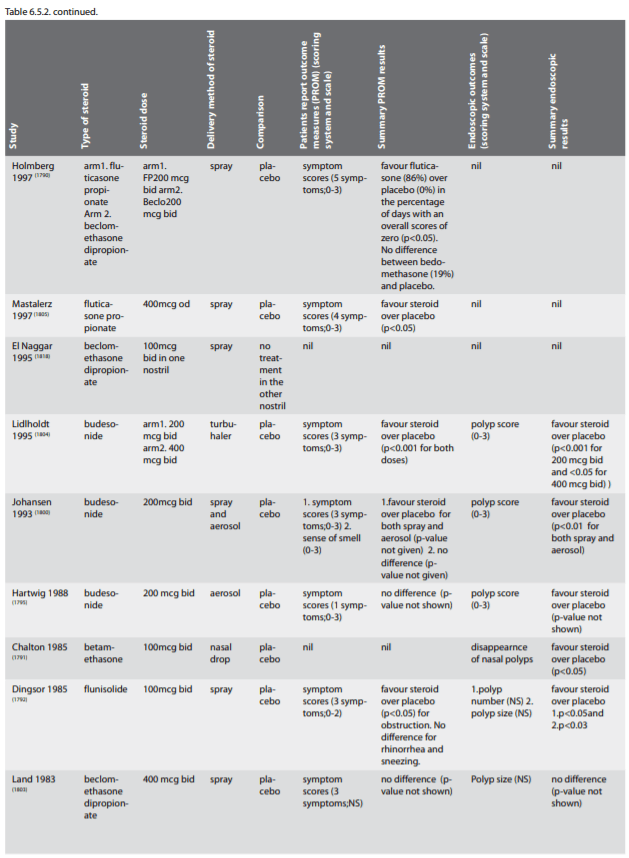
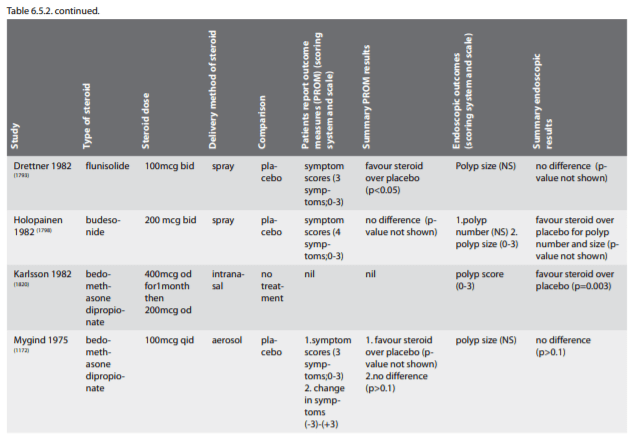
6.5.2.7.1. Effect of prior surgery
Patients with sinus surgery responded to topical steroid greater than patients without sinus surgery in polyp size reduction (Figure 6.5.5.). However improvement in symptoms and nasal airflow was not statistically different between the two subgroups (Figure 6.5.6.). It is difficult to make a complete assessment as not all studies could be pooled for meta-analysis. A summary of studies that showed a benefit with INCS by the surgical status of their patient population is shown in Table 6.5.3.
6.5.2.7.2. Effect of delivery of spray v drops
Nasal aerosols and turbuhaler were found more effective than nasal spray in symptom control (Figure 6.5.7.) but there was no difference in polyp size reduction and nasal airway across various types of topical delivery methods. Similar to assessing the surgical state, a complete assessment is difficult as not all studies could be pooled for meta-analysis. A summary of studies that showed a benefit with INCS by spray or drop is shown in Table 6.5.4. No study reported on direct sinus delivery methods or high volume, high pressure delivery in patient with prior sinus surgery.
6.5.2.7.3. Effect of modern corticosteroid v first generation
There does not appear to be a significant benefit of modern corticosteroid against first-generation for the final symptom score (Figure 6.5.8.a) or for responder with polyp reduction (Figure 6.5.8.b).
6.5.2.8. Side-effects of local corticosteroid chronic rhinosinusitis with nasal polyps
The most common events were epistaxis and nasal irritation including itching, sneeze, dry nose and rhinitis. Adverse events reported were possibly ambiguous. Rhinitis symptoms could be disease-related. It is acknowledge that rare adverse events are possibly not detected in randomised controlled trials (RCTs). However, they were extremely low and there was no difference in adverse events between the study groups and control groups in any trial. Post-market adverse events for intranasal steroid sprays are very low. However, we have not specifically sought adverse event data from non-RCT studies. Minor adverse events from nasal steroids are commonly tolerated by patients. The amount of benefit clearly outweighs the risk. The reported adverse events from the included studies are summarized in Table 6.5.5.
Reported epistaxis may be attributable to local effects of the INCS on septal mucosa and exacerbated by poor technique (1826) with significance preponderance of the side of epistaxis to handedness. Some have attributed epistaxis to the vasoconstrictor activity (1827) of the corticosteroid molecules, and postulated this as a mechanism for the very rare occurrence of nasal septal perforation (1828). However, it should be remembered that minor nose bleeds are common in the population, occurring in 16.5% of 2197 women aged 50-64 years over a one year study (1829) and that spontaneous nasal perforation occurs within the community at a low rate (1830). Nasal biopsy studies do not show any detrimental structural effects within the nasal mucosa with long-term administration of intranasal corticosteroids and atrophy does not occur as the mucosa is a single layer of epithelium compared to keratin producing multi-layered skin where atrophy is reported (1831- 1838). Much attention has focused on the systemic safety of intranasal application. The systemic bioavailability of intranasal corticosteroids varies from <1% to up to 40-50% and influences the risk of systemic adverse effects (1828, 1839). Potential adverse events related to the administration of intranasal corticosteroids are effects on growth, ocular effects, effects on bone, and on the hypothalamic-pituitary-adrenal axis (1840). Because the dose delivered topically is small, this is not a major consideration, and extensive studies have not identified significant effects on the hypothalamic-pituitary-adrenal axis with continued treatment. A small effect on growth has been reported in one study in children receiving a standard dosage over 1 year. However, this has not been found in prospective studies with the intranasal corticosteroids that have low systemic bioavailability and therefore the judicious choice of intranasal formulation, particularly if there is concurrent corticosteroid inhalation for asthma, is prudent (1841). In summary, intranasal corticosteroids are highly effective; nevertheless, they are not completely devoid of systemic effects. Thus, care has to be taken, especially in children, when long-term treatments are prescribed. However the systemic effects of nasal corticosteroids are negligible compared to inhaled corticosteroids.
Patients with sinus surgery responded to topical steroid greater than patients without sinus surgery in polyp size reduction (Figure 6.5.5.). However improvement in symptoms and nasal airflow was not statistically different between the two subgroups (Figure 6.5.6.). It is difficult to make a complete assessment as not all studies could be pooled for meta-analysis. A summary of studies that showed a benefit with INCS by the surgical status of their patient population is shown in Table 6.5.3.
6.5.2.7.2. Effect of delivery of spray v drops
Nasal aerosols and turbuhaler were found more effective than nasal spray in symptom control (Figure 6.5.7.) but there was no difference in polyp size reduction and nasal airway across various types of topical delivery methods. Similar to assessing the surgical state, a complete assessment is difficult as not all studies could be pooled for meta-analysis. A summary of studies that showed a benefit with INCS by spray or drop is shown in Table 6.5.4. No study reported on direct sinus delivery methods or high volume, high pressure delivery in patient with prior sinus surgery.
6.5.2.7.3. Effect of modern corticosteroid v first generation
There does not appear to be a significant benefit of modern corticosteroid against first-generation for the final symptom score (Figure 6.5.8.a) or for responder with polyp reduction (Figure 6.5.8.b).
6.5.2.8. Side-effects of local corticosteroid chronic rhinosinusitis with nasal polyps
The most common events were epistaxis and nasal irritation including itching, sneeze, dry nose and rhinitis. Adverse events reported were possibly ambiguous. Rhinitis symptoms could be disease-related. It is acknowledge that rare adverse events are possibly not detected in randomised controlled trials (RCTs). However, they were extremely low and there was no difference in adverse events between the study groups and control groups in any trial. Post-market adverse events for intranasal steroid sprays are very low. However, we have not specifically sought adverse event data from non-RCT studies. Minor adverse events from nasal steroids are commonly tolerated by patients. The amount of benefit clearly outweighs the risk. The reported adverse events from the included studies are summarized in Table 6.5.5.
Reported epistaxis may be attributable to local effects of the INCS on septal mucosa and exacerbated by poor technique (1826) with significance preponderance of the side of epistaxis to handedness. Some have attributed epistaxis to the vasoconstrictor activity (1827) of the corticosteroid molecules, and postulated this as a mechanism for the very rare occurrence of nasal septal perforation (1828). However, it should be remembered that minor nose bleeds are common in the population, occurring in 16.5% of 2197 women aged 50-64 years over a one year study (1829) and that spontaneous nasal perforation occurs within the community at a low rate (1830). Nasal biopsy studies do not show any detrimental structural effects within the nasal mucosa with long-term administration of intranasal corticosteroids and atrophy does not occur as the mucosa is a single layer of epithelium compared to keratin producing multi-layered skin where atrophy is reported (1831- 1838). Much attention has focused on the systemic safety of intranasal application. The systemic bioavailability of intranasal corticosteroids varies from <1% to up to 40-50% and influences the risk of systemic adverse effects (1828, 1839). Potential adverse events related to the administration of intranasal corticosteroids are effects on growth, ocular effects, effects on bone, and on the hypothalamic-pituitary-adrenal axis (1840). Because the dose delivered topically is small, this is not a major consideration, and extensive studies have not identified significant effects on the hypothalamic-pituitary-adrenal axis with continued treatment. A small effect on growth has been reported in one study in children receiving a standard dosage over 1 year. However, this has not been found in prospective studies with the intranasal corticosteroids that have low systemic bioavailability and therefore the judicious choice of intranasal formulation, particularly if there is concurrent corticosteroid inhalation for asthma, is prudent (1841). In summary, intranasal corticosteroids are highly effective; nevertheless, they are not completely devoid of systemic effects. Thus, care has to be taken, especially in children, when long-term treatments are prescribed. However the systemic effects of nasal corticosteroids are negligible compared to inhaled corticosteroids.
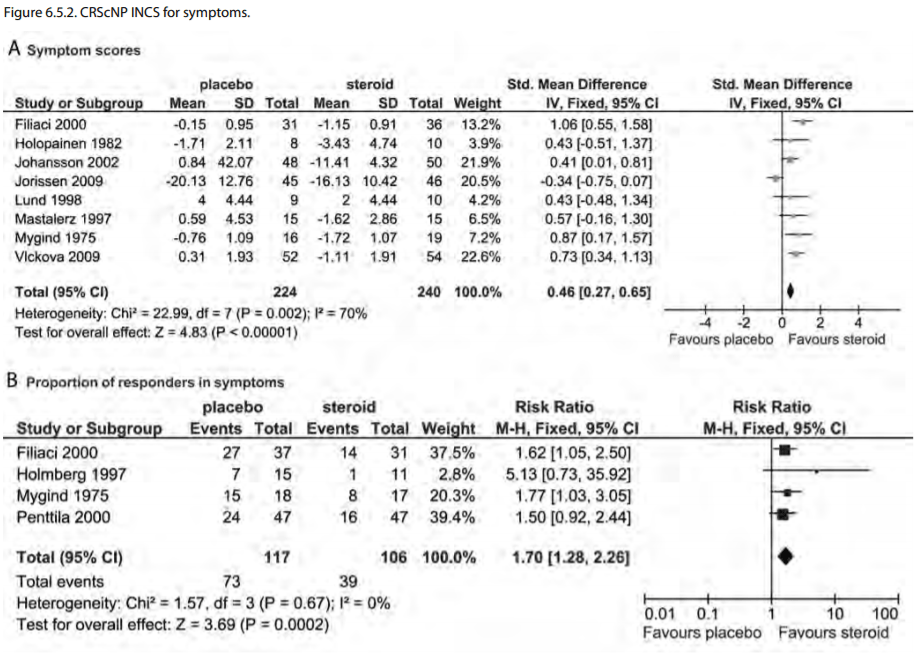
6.5.3. Systemic corticosteroid chronic rhinosinusitis with
nasal polyps
Traditionally systemic steroids have been used in patients based on the significant effect on NP supported by open studies where a single injection of 14 mg betametasone have been compared with snare polypectomy surgery (1085, 1842). In these studies effects are seen on nasal polyp size, nasal symptom score and nasal expiratory peak flow but it is difficult to differentiate the effect of systemic steroids from that of local treatment since both treatments were used at the same time. The control groups underwent surgery during the study period. Since then a Cochrane review published in 2007 and its update in October 2010 has identified 3 level 1 studies (166 patients) to support the use of systemic corticosteroids in CRS with nasal polyps. The characteristics of these 3 included studies including the addition of 3 additional studies are described in Table 6.5.6.
Martinez-Anton 2008 (1843) and Benitez 2006 (1169) both contain the same data on the randomized component of these trials with oral corticosteroid (author correspondence).. The two other trials include a RCT in the allergic fungal sinusitis subtype of CRSwNP (1572) and a recent study of pre-treatment with and without systemic corticosteroid before ongoing INCS (1844). There is definite intermediate effect that occurs with both symptoms and polyp size (Table 6.5.7.). However, given the inherent short period that this therapy is applied in a chronic condition, the treatment effects are short lived.
A combined oral followed by INCS protocol was described by Benitez et al.(1169) who performed a randomized placebo controlled study with prednisone for two weeks (30 mg 4 days followed by a 2-day reduction of 5 mg). After two weeks on prednisone or placebo, the prednisone group continued for ten weeks on intranasal BUD. After two weeks treatment a significant polyp reduction was seen, several symptoms improved and anterior rhinomanometry improved compared to the placebo group. After 12 weeks a significant reduction of CT-changes were seen in the steroid treated group.
Traditionally systemic steroids have been used in patients based on the significant effect on NP supported by open studies where a single injection of 14 mg betametasone have been compared with snare polypectomy surgery (1085, 1842). In these studies effects are seen on nasal polyp size, nasal symptom score and nasal expiratory peak flow but it is difficult to differentiate the effect of systemic steroids from that of local treatment since both treatments were used at the same time. The control groups underwent surgery during the study period. Since then a Cochrane review published in 2007 and its update in October 2010 has identified 3 level 1 studies (166 patients) to support the use of systemic corticosteroids in CRS with nasal polyps. The characteristics of these 3 included studies including the addition of 3 additional studies are described in Table 6.5.6.
Martinez-Anton 2008 (1843) and Benitez 2006 (1169) both contain the same data on the randomized component of these trials with oral corticosteroid (author correspondence).. The two other trials include a RCT in the allergic fungal sinusitis subtype of CRSwNP (1572) and a recent study of pre-treatment with and without systemic corticosteroid before ongoing INCS (1844). There is definite intermediate effect that occurs with both symptoms and polyp size (Table 6.5.7.). However, given the inherent short period that this therapy is applied in a chronic condition, the treatment effects are short lived.
A combined oral followed by INCS protocol was described by Benitez et al.(1169) who performed a randomized placebo controlled study with prednisone for two weeks (30 mg 4 days followed by a 2-day reduction of 5 mg). After two weeks on prednisone or placebo, the prednisone group continued for ten weeks on intranasal BUD. After two weeks treatment a significant polyp reduction was seen, several symptoms improved and anterior rhinomanometry improved compared to the placebo group. After 12 weeks a significant reduction of CT-changes were seen in the steroid treated group.
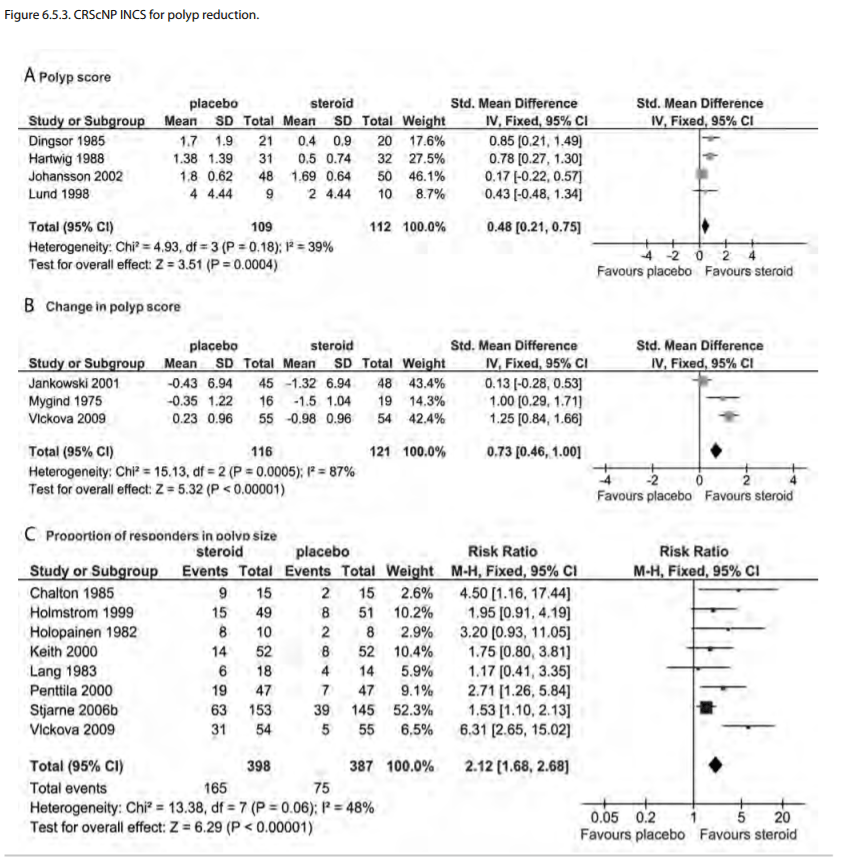
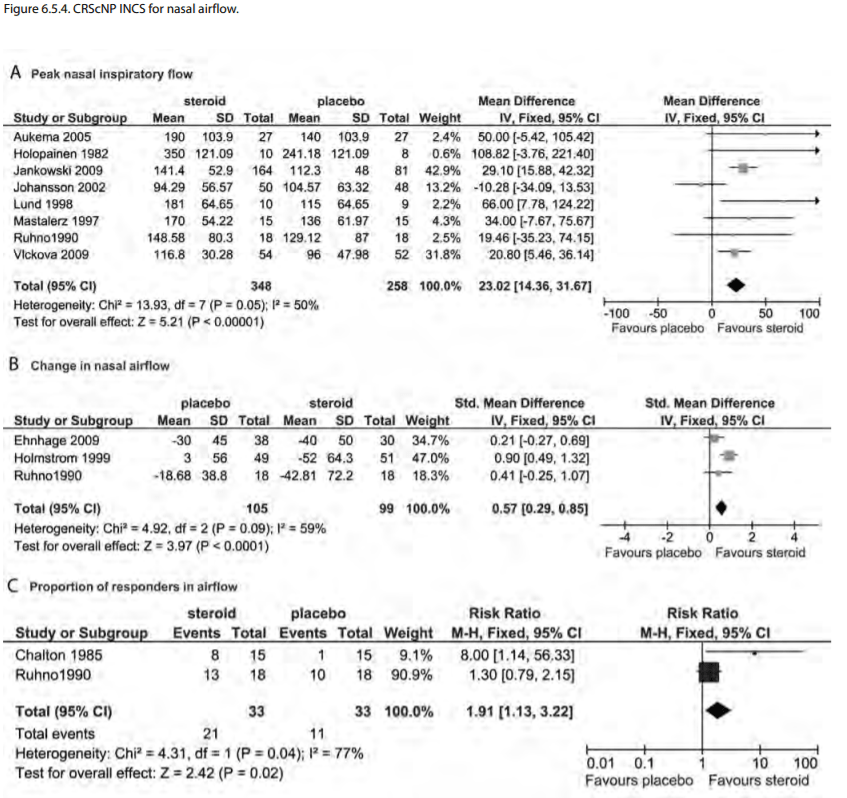
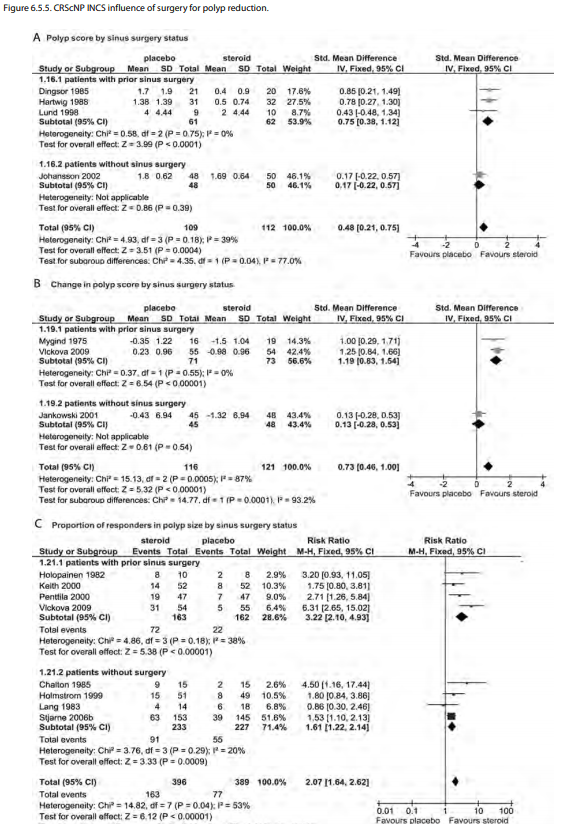
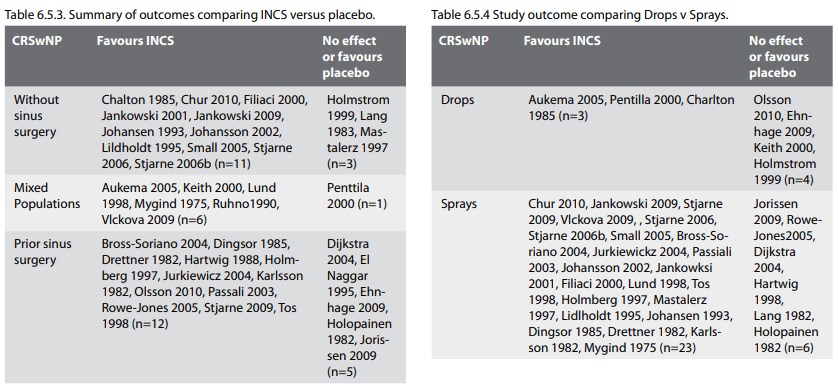
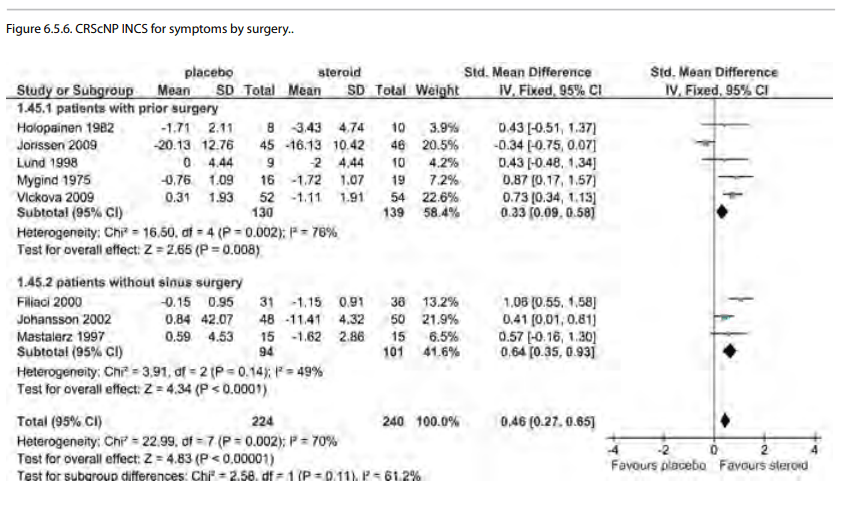
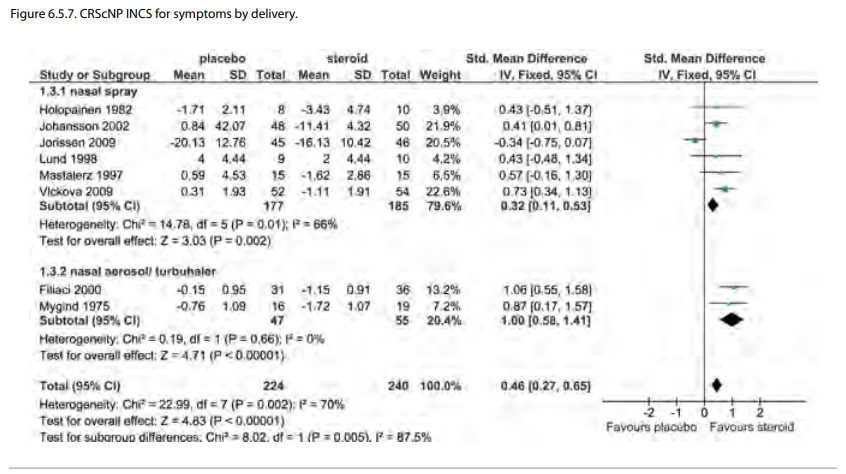
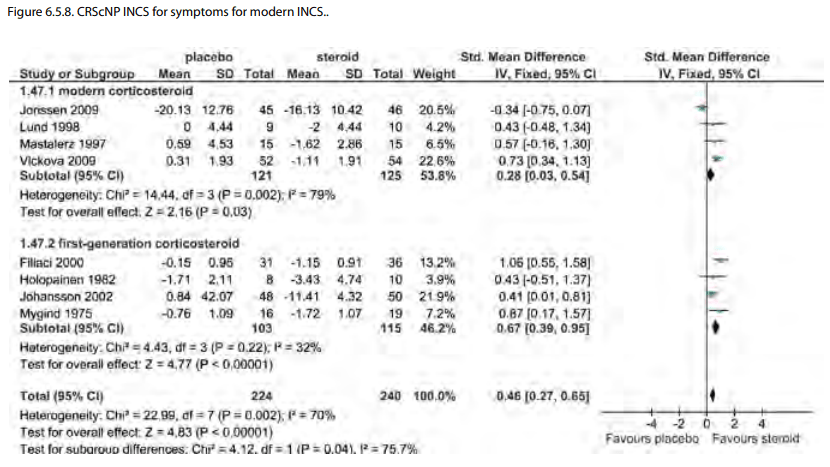
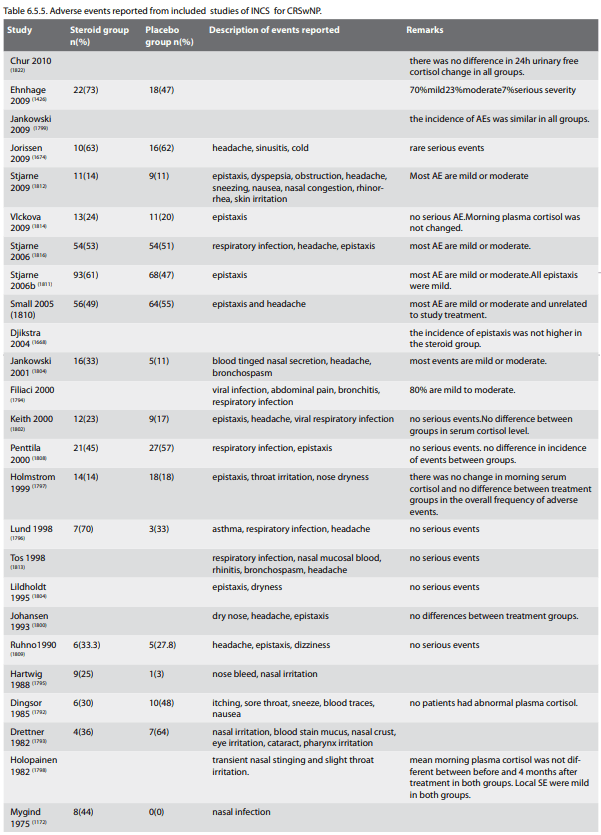
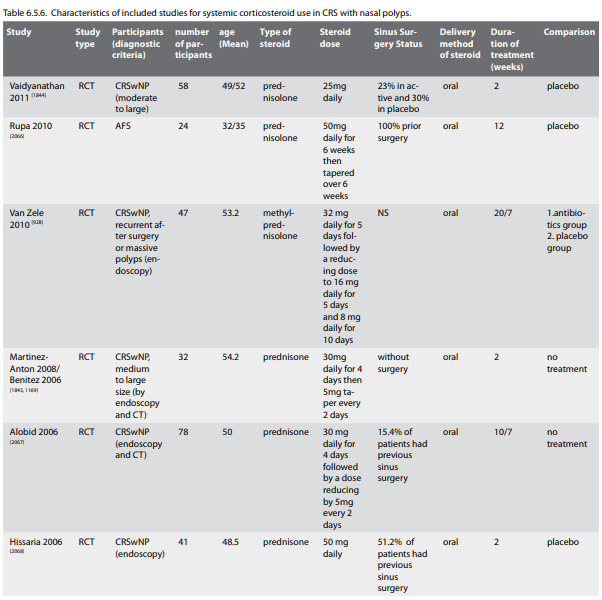

6.5.3.1. Side-effects of systemic corticosteroid chronic
rhinosinusitis with nasal polyps
The anti-inflammatory effects of corticosteroids cannot be separated from their metabolic effects as all cells use the same glucocorticoid receptor; therefore when corticosteroids are prescribed measures should be taken to minimize their side effects. Clearly, the chance of significant side effects increases with the dose and duration of treatment and so the minimum dose necessary to control the disease should be given. Patients on systemic corticosteroid therapy should be aware of impact on bone mineral density and regular calcium+vitamin D supplements are recommended. A bone density study every two years is commonly performed. There are changes to fat metabolism, catabolic muscle effects and appetite changes such that careful diet, exercise and weight management should be instituted. Additionally, the impact on glucose tolerance, early cataract formation and the pituitary-hypothalmic axis suppression need to be assessed and the patient educated on the impact of these.
6.5.4. Evidence based recommendations
There is good evidence that both INCS and systemic corticosteroids are effective for the management of CRSwNP. However, considering the evolving understanding of CRSwNP and the chronicity of this condition (not from lack of treatment but natural history) many treatments will need to ongoing similar to local corticosteroid therapy in asthma. Thus the short-lived benefits of systemic corticosteroid therapy need to be balanced with the long-term potential side-effects. Local therapy appears to be effective but the ability to effectively deliver INCS to the paranasal sinuses may greatly influence the treatment response.
The anti-inflammatory effects of corticosteroids cannot be separated from their metabolic effects as all cells use the same glucocorticoid receptor; therefore when corticosteroids are prescribed measures should be taken to minimize their side effects. Clearly, the chance of significant side effects increases with the dose and duration of treatment and so the minimum dose necessary to control the disease should be given. Patients on systemic corticosteroid therapy should be aware of impact on bone mineral density and regular calcium+vitamin D supplements are recommended. A bone density study every two years is commonly performed. There are changes to fat metabolism, catabolic muscle effects and appetite changes such that careful diet, exercise and weight management should be instituted. Additionally, the impact on glucose tolerance, early cataract formation and the pituitary-hypothalmic axis suppression need to be assessed and the patient educated on the impact of these.
6.5.4. Evidence based recommendations
There is good evidence that both INCS and systemic corticosteroids are effective for the management of CRSwNP. However, considering the evolving understanding of CRSwNP and the chronicity of this condition (not from lack of treatment but natural history) many treatments will need to ongoing similar to local corticosteroid therapy in asthma. Thus the short-lived benefits of systemic corticosteroid therapy need to be balanced with the long-term potential side-effects. Local therapy appears to be effective but the ability to effectively deliver INCS to the paranasal sinuses may greatly influence the treatment response.
6.6. Treatment CRSwNP with antibiotics
Systemic doxycycline treatment for 3 weeks reduce polyp size and post-nasal discharge but not other symptoms compared to placebo in CRSwP.
6.6.1. Short-term treatment with antibiotics in CRSwNP
Short-term treatment with antibiotics in chronic rhinosinusitis with polyps
Two recent placebo controlled studies are available. It is the theory of endotoxin producing staphylococci as disease modifiers in CRSwNP that has prompted the interest. A placebocontrolled study by van Zele and co-workers, compared the effect of methylprednisolone in a 3 week course (32 mg for 1 w, 16 mg for 1 week and finally 8 mg for 1 week) with doxycycline (100 mg except for the first day of 200 mg) for 20 days with placebo.
Another placebo controlled study was performed by Schalek and co-authors (1845) 23 patients undergoing FESS, who tested positive for S. Aureus enterotoxin producing strains were randomized to oral anti-staphylococcal antibiotics (quinolone, amoxicillin/clavulanate or co-trimoxazole) for 3 weeks, or placebo. Both groups were compared pre-operatively and at 3 and 6 months using endoscopic score and SNOT-22. Slightly better results were found in the antibiotic group but it did not reach significance. Inflammatory markers were measured in both nasal secretions and blood, polyp size was estimated and symptoms were registered. Methylprednisolone had a short but dramatic effect on polyp size and symptoms. Doxycycline had a significant but small effect on polyp size compared to placebo, which was present for the length of the study, 12 weeks. Doxycycline showed a significant effect on postnasal discharge leaving other symptoms unchanged. Analysis of nasal secretions revealed that doxycycline reduced metallomatrix protein-9 (MMP-9) as well as myeoloperoxidase (MPO) and eosinophilic cationic protein (ECP). However quality of life measurements are lacking and one cannot deduce from the results whether the effect of doxycycline improved quality of life in the study population (928).
Conclusion
One RCT have shown that doxycycline for 3 weeks had a small effect on polyp size and post-nasal discharge but not other symptoms compared to placebo. The second study, where sample size was low, showed a trend towards effect (Level of evidence 1b) (Recommendation C).
Systemic doxycycline treatment for 3 weeks reduce polyp size and post-nasal discharge but not other symptoms compared to placebo in CRSwP.
6.6.1. Short-term treatment with antibiotics in CRSwNP
Short-term treatment with antibiotics in chronic rhinosinusitis with polyps
Two recent placebo controlled studies are available. It is the theory of endotoxin producing staphylococci as disease modifiers in CRSwNP that has prompted the interest. A placebocontrolled study by van Zele and co-workers, compared the effect of methylprednisolone in a 3 week course (32 mg for 1 w, 16 mg for 1 week and finally 8 mg for 1 week) with doxycycline (100 mg except for the first day of 200 mg) for 20 days with placebo.
Another placebo controlled study was performed by Schalek and co-authors (1845) 23 patients undergoing FESS, who tested positive for S. Aureus enterotoxin producing strains were randomized to oral anti-staphylococcal antibiotics (quinolone, amoxicillin/clavulanate or co-trimoxazole) for 3 weeks, or placebo. Both groups were compared pre-operatively and at 3 and 6 months using endoscopic score and SNOT-22. Slightly better results were found in the antibiotic group but it did not reach significance. Inflammatory markers were measured in both nasal secretions and blood, polyp size was estimated and symptoms were registered. Methylprednisolone had a short but dramatic effect on polyp size and symptoms. Doxycycline had a significant but small effect on polyp size compared to placebo, which was present for the length of the study, 12 weeks. Doxycycline showed a significant effect on postnasal discharge leaving other symptoms unchanged. Analysis of nasal secretions revealed that doxycycline reduced metallomatrix protein-9 (MMP-9) as well as myeoloperoxidase (MPO) and eosinophilic cationic protein (ECP). However quality of life measurements are lacking and one cannot deduce from the results whether the effect of doxycycline improved quality of life in the study population (928).
Conclusion
One RCT have shown that doxycycline for 3 weeks had a small effect on polyp size and post-nasal discharge but not other symptoms compared to placebo. The second study, where sample size was low, showed a trend towards effect (Level of evidence 1b) (Recommendation C).
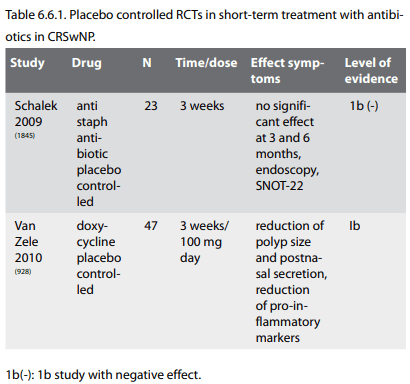
6.6.2. Long-term treatment with antibiotics in CRSwNP
There are few studies where the study population has been properly defined into groups with, or without polyps. However one can identify at least 3 open studies where effect on polyp size is mentioned.
In an uncontrolled trial twenty patients with CRS and nasal polyps were treated for at least 3 months with clarithromycin 400 mg/day. In the group whose polyps were reduced in size, the IL-8 levels decreased and were initially significantly higher before macrolide treatment than those in the group whose polyps showed no change (1846). In another uncontrolled trial 40 patients altogether were treated with either roxithromycin 150 mg alone or in combination with an antihistamine (azelastine) for at least 8 weeks. Smaller polyps were more likely to shrink and this happened in about half of the patients (1702). A small, n=12, open study, using Roxithromycin 150 mg x1, also showed a reduction in IL-8 and improved aeration on CT (1704).
Conclusion
A few open studies have shown some effect on polyp size and patient symptoms. The effect seems to be moderate but may be more long lasting than systemic steroids, however quality of life data are missing and the clinical benefit for the patient is not fully investigated to date. Further studies are necessary to evaluate this treatment option (Level of evidence III. Strength of recommendation C).
There are few studies where the study population has been properly defined into groups with, or without polyps. However one can identify at least 3 open studies where effect on polyp size is mentioned.
In an uncontrolled trial twenty patients with CRS and nasal polyps were treated for at least 3 months with clarithromycin 400 mg/day. In the group whose polyps were reduced in size, the IL-8 levels decreased and were initially significantly higher before macrolide treatment than those in the group whose polyps showed no change (1846). In another uncontrolled trial 40 patients altogether were treated with either roxithromycin 150 mg alone or in combination with an antihistamine (azelastine) for at least 8 weeks. Smaller polyps were more likely to shrink and this happened in about half of the patients (1702). A small, n=12, open study, using Roxithromycin 150 mg x1, also showed a reduction in IL-8 and improved aeration on CT (1704).
Conclusion
A few open studies have shown some effect on polyp size and patient symptoms. The effect seems to be moderate but may be more long lasting than systemic steroids, however quality of life data are missing and the clinical benefit for the patient is not fully investigated to date. Further studies are necessary to evaluate this treatment option (Level of evidence III. Strength of recommendation C).
6.6.3. Treatment with topical antibiotics in CRSwNP
There are no data on the effect of topical antibitoics in CRSwNP.
6.6.4. Adverse events of antibiotic therapy of CRS
6.6.4.1. Effects on bacterial resistance.
A concern with long-term antibacterial treatment is the emergence of resistant bacterial strains. Especially when using a low dose not attaining minimal inhibitory concentrations. Data from primary care have shown that increased macrolide prescription in group A streptococci tonsillitis leads to a subsequent increase in resistance, which can reach alarmingly high levels (1714, 1715). However in a tertiary setting, data is sketchy. The study by Videler at al. using azithromycin for 12 weeks, found 3 of 50 cultures with macrolide resistant strains before treatment, and after treatment 4 of 43 cultures with resistant strains (1709). An emerging concern in cystic fibrosis patients is the increasing incidence of infection with the highly pathogenic Mycobacterium abscessus in azithromycin treated patients. The effect is probably due to azithromycin inhibition of autophagic and phagosomal degradation (1716-1718). This has not been reported in CRS patients.
6.6.4.2. Other side effects
Well-known side effects of antibiotics includes: gastrointestinal upset, skin rash reversible elevation of liver enzymes. In the study by Videler et al. including 78 patients, the investigators found 1 case of muscle ache in the azithroprim group and 2 cases of mild skin rash in the clarithromycin treated patients and no adverse effects in the trimethroprim-sulfamethoxazole group. The study comparing doxycycline treatment for 20 days with methylprednisolone and placebo reported no difference in adverse events in the different groups. However, rare side effects are not picked up in small clinical trials, but rather in national records on side effects. Hearing impairment due to macrolide treatment is a rare side effect but was recorded in a recent large trial in COPD (1696).
6.6.4.3. Conclusions on adverse events of antibiotic therapy of CRS
The safety of long-term antibiotic therapy, either azithromycin, clarithromycin or roxithromycin is recognised in patients with CRS, but also due to it's established long-term use in cystic fibrosis. As for doxycycline there is longstanding experience for long-term use in acne and rosacea patients. Trimethroprimsulfamethoxazole has been used long-term in both the pediatric and adult population for treatment of infectious prone patients with certain immune deficiencies as well as urinary tract infections. Drawing on the experience from other areas than CRS, long-term treatment with the mentioned antibiotics is relatively safe. Although one has to bear in mind the interaction between macrolides and drugs such as dicumarol, antiepileptic drugs, terphenadine, methotrexate and antidepressant drugs. To monitor the risk of the development of resistant bacterial strains, nasal swabs with culture every 3 months during treatment is advisable.
There are no data on the effect of topical antibitoics in CRSwNP.
6.6.4. Adverse events of antibiotic therapy of CRS
6.6.4.1. Effects on bacterial resistance.
A concern with long-term antibacterial treatment is the emergence of resistant bacterial strains. Especially when using a low dose not attaining minimal inhibitory concentrations. Data from primary care have shown that increased macrolide prescription in group A streptococci tonsillitis leads to a subsequent increase in resistance, which can reach alarmingly high levels (1714, 1715). However in a tertiary setting, data is sketchy. The study by Videler at al. using azithromycin for 12 weeks, found 3 of 50 cultures with macrolide resistant strains before treatment, and after treatment 4 of 43 cultures with resistant strains (1709). An emerging concern in cystic fibrosis patients is the increasing incidence of infection with the highly pathogenic Mycobacterium abscessus in azithromycin treated patients. The effect is probably due to azithromycin inhibition of autophagic and phagosomal degradation (1716-1718). This has not been reported in CRS patients.
6.6.4.2. Other side effects
Well-known side effects of antibiotics includes: gastrointestinal upset, skin rash reversible elevation of liver enzymes. In the study by Videler et al. including 78 patients, the investigators found 1 case of muscle ache in the azithroprim group and 2 cases of mild skin rash in the clarithromycin treated patients and no adverse effects in the trimethroprim-sulfamethoxazole group. The study comparing doxycycline treatment for 20 days with methylprednisolone and placebo reported no difference in adverse events in the different groups. However, rare side effects are not picked up in small clinical trials, but rather in national records on side effects. Hearing impairment due to macrolide treatment is a rare side effect but was recorded in a recent large trial in COPD (1696).
6.6.4.3. Conclusions on adverse events of antibiotic therapy of CRS
The safety of long-term antibiotic therapy, either azithromycin, clarithromycin or roxithromycin is recognised in patients with CRS, but also due to it's established long-term use in cystic fibrosis. As for doxycycline there is longstanding experience for long-term use in acne and rosacea patients. Trimethroprimsulfamethoxazole has been used long-term in both the pediatric and adult population for treatment of infectious prone patients with certain immune deficiencies as well as urinary tract infections. Drawing on the experience from other areas than CRS, long-term treatment with the mentioned antibiotics is relatively safe. Although one has to bear in mind the interaction between macrolides and drugs such as dicumarol, antiepileptic drugs, terphenadine, methotrexate and antidepressant drugs. To monitor the risk of the development of resistant bacterial strains, nasal swabs with culture every 3 months during treatment is advisable.
6.7. Other medical management in CRSwNP
6.7.1. Summary
Current data yield insufficient evidence to recommend anti-IgE, anti-IL5, antihistamines in non-allergic patients, antimycotics, immunosuppressants, furosemide, leukotriene antagonists, aspirin desensitisation, capsaicin and various other medical treatments for treatment of CRSwNP.
6.7.2. Introduction
In the EP3 OS 2007 publication (8), non-antibiotic and nonsteroidal medical treatment of acute rhinosinusitis, CRSsNP and CRSwNP were discussed in one chapter. Due to differences in aetiology and pathophysiology, but also treatment principles, the authors decided to consider CRSwNP and CRSsNP in adults in separate chapters. Criteria to include publications in the current analysis were more restrictive, mainly focused on randomized controlled trials (RCT) and studies published after 2007. As a consequence, not all publications cited in the former version of EPOS were included. An analysis of publications on anti-IgE and anti-IL-5 antibodies was included. Moreover, a more in depth analysis of included publications was performed. The part on antihistamines was revised. The part on topical amphotericin B treatment was replaced due to the availability of a recent comprehensive Cochrane analysis. Tables were restricted to RCT, when at least two trials were available. A column was added indicating if patients with or without previous sinus surgery were included. For each substance group, most relevant adverse effects and levels of recommendation are provided.
6.7.3. Anti-IgE
In several investigations, total IgE-levels in nasal secretions, nasal polyp homogenisates and blood serum were higher in CRS-patients with nasal polyps than in controls. Omalizumab(R), a recombinant DNA-derived humanized IgG1k monoclonal antibody that selectively binds to human IgE, reduces serum and tissue IgE-levels. Omalizumab(R) is approved for patients with moderate-to-severe or severe allergic asthma. Two anecdotal reports (1847, 1848), 1 pilot study in 8 patients (1849) and 1 case series (1850) showed beneficial effects of omalizumab(R) in CRS patients with nasal polyps. Pinto and co-workers conducted a randomized, double-blind, placebo-controlled trial of antiIgE for chronic rhinosinusitis in 14 patients (12/14 with nasal polyps) with severe CRS refractory to standard treatment including sinus surgery (963). Pretreatment serum total IgE-levels between 30 - 700 IU/ml were required for inclusion. All patients received omalizumab(R) 0.016 mg/kg per IU total serum IgE/ mL subcutaneously or placebo injections all 4 weeks for 6 months on top og other medical treatment. The main outcome parameter was pre- and post-treatment sinus opacification in coronal CT scans. The median change of sinus opacification in omalizumab(R) treated patients was 11.9% vs. 5.9% in placebo treated patients (p<0.391). No significant differences were also found in various secondary outcome parameters including SNOT-20 scores, olfactory test scores, endoscopy scores, eosinophils in nasal lavage, and peak nasal inspiratory flow values. This study is underpowered due to recruitment problems following FDA warnings on anaphylactic AE to omalizumab. Omalizumab may cause anaphylaxis in approximately 1 patient per 1,000 (1851). Omalizumab may increase the risk of cardiovascular events, thrombocytopenia or cancer (1852). Based on current data, omalizumab is not recommended for the treatment of CRS with nasal polyps (grade of recommendation: C). Mainly data on patients with previous sinus surgery are available.
6.7.4. Anti-IL-5
IL-5 is a key activator in eosinophil growth, recruitment and activation. High amounts of IL-5 were detected in polyp homogenisates, nasal secretions and blood serum of patients with NP. Mepolizumab (Glaxo Smith Kline) and reslizumab (Schering-Plough) are humanized anti–IL-5 mAb that reduce the number of eosinophils in blood and tissues (1853, 1854). Both antibodies currently undergo Phase II and III trials. In 2004, orphan designation (EU/3/04/213) was granted by the European Commission for mepolizumab for the treatment of hypereosinophilic syndrome. Two clinical trials with anti-IL-5 antibodies in CRS patients with nasal polyps were identified.
In a double-blind, placebo-controlled, randomized, 2-center phase I/II trial, 24 subjects with bilateral nasal polyps were randomized to receive a single intravenous infusion of reslizumab 3mg/kg or 1mg/kg or placebo (i.e. 8 patients per treatment arm). The post-injection observation period was 36 weeks. Adverse events did not significantly differ between treatment groups. No pharmacokinetic data and no detailed data on drop outs are provided. Main outcome measure for efficacy was an endoscopic nasal polyp score repeatedly evaluated for each nostril. Secondary efficacy parameters included peak nasal inspiratory airflow and nasal symptom scores. At no individual time point, a significant difference in the symptom scores or in the nasal peak inspiratory flow values was observed in both treatment groups compared with those in the placebo group. The total nasal polyp score was significantly decreased in the 1mg/kg group at week 12 compared with baseline values, however apparently not with the values of the control group. No dose response relation was observed. Blood eosinophil counts dropped significantly in both active groups, followed by a steep increase above baseline values 8-19 weeks post injection suggesting a rebound hypereosinophilia. Patients with nasal secretion IL-5 levels >40pg/ml were more likely to reveal a reduction of at least 1 polyp score on an 8 point scale when treated with anti-IL-5 (931).
In a further randomized, double-blind, placebo-controlled study, CRS-patients with nasal polyps received 2 single intravenous injections (28 days apart) of 750 mg of mepolizumab (20 subjects) or placebo (10 subjects). Post-injection observation period was 48 weeks. The primary end point was the reduction in an endoscopic nasal polyp score on an 8 point scale at 8 weeks after the first dosing (4 weeks after the second dose). Secondary outcome parameter included sinus opacification in CT scans, peak nasal inspiratory flow and symptom scores. Last observation carried forward imputation was used to handle missing data. Number and severity of adverse events did not differ between treatment groups. In the treatment group, nasal polyp scores improved 1.30±1.72 (SD) score points while it remained unchanged (0.00±0.94) in the control group, resulting in a treatment difference of 1.30±1.51 score points (p=0.028, Mann-Whitney U test). Moreover, significantly less sinus opacification was observed in the treatment arm (932).
The results of these clinical trials suggest that anti-IL-5 antibodies could play a role in the treatment of selected CRS-patients with nasal polyps. In a recent reslizumab study in asthma patients, nasopharyngitis, fatigue, and pharyngolaryngeal pain were common adverse events (1855). No data on patients without previous sinus surgery are available.
6.7.1. Summary
Current data yield insufficient evidence to recommend anti-IgE, anti-IL5, antihistamines in non-allergic patients, antimycotics, immunosuppressants, furosemide, leukotriene antagonists, aspirin desensitisation, capsaicin and various other medical treatments for treatment of CRSwNP.
6.7.2. Introduction
In the EP3 OS 2007 publication (8), non-antibiotic and nonsteroidal medical treatment of acute rhinosinusitis, CRSsNP and CRSwNP were discussed in one chapter. Due to differences in aetiology and pathophysiology, but also treatment principles, the authors decided to consider CRSwNP and CRSsNP in adults in separate chapters. Criteria to include publications in the current analysis were more restrictive, mainly focused on randomized controlled trials (RCT) and studies published after 2007. As a consequence, not all publications cited in the former version of EPOS were included. An analysis of publications on anti-IgE and anti-IL-5 antibodies was included. Moreover, a more in depth analysis of included publications was performed. The part on antihistamines was revised. The part on topical amphotericin B treatment was replaced due to the availability of a recent comprehensive Cochrane analysis. Tables were restricted to RCT, when at least two trials were available. A column was added indicating if patients with or without previous sinus surgery were included. For each substance group, most relevant adverse effects and levels of recommendation are provided.
6.7.3. Anti-IgE
In several investigations, total IgE-levels in nasal secretions, nasal polyp homogenisates and blood serum were higher in CRS-patients with nasal polyps than in controls. Omalizumab(R), a recombinant DNA-derived humanized IgG1k monoclonal antibody that selectively binds to human IgE, reduces serum and tissue IgE-levels. Omalizumab(R) is approved for patients with moderate-to-severe or severe allergic asthma. Two anecdotal reports (1847, 1848), 1 pilot study in 8 patients (1849) and 1 case series (1850) showed beneficial effects of omalizumab(R) in CRS patients with nasal polyps. Pinto and co-workers conducted a randomized, double-blind, placebo-controlled trial of antiIgE for chronic rhinosinusitis in 14 patients (12/14 with nasal polyps) with severe CRS refractory to standard treatment including sinus surgery (963). Pretreatment serum total IgE-levels between 30 - 700 IU/ml were required for inclusion. All patients received omalizumab(R) 0.016 mg/kg per IU total serum IgE/ mL subcutaneously or placebo injections all 4 weeks for 6 months on top og other medical treatment. The main outcome parameter was pre- and post-treatment sinus opacification in coronal CT scans. The median change of sinus opacification in omalizumab(R) treated patients was 11.9% vs. 5.9% in placebo treated patients (p<0.391). No significant differences were also found in various secondary outcome parameters including SNOT-20 scores, olfactory test scores, endoscopy scores, eosinophils in nasal lavage, and peak nasal inspiratory flow values. This study is underpowered due to recruitment problems following FDA warnings on anaphylactic AE to omalizumab. Omalizumab may cause anaphylaxis in approximately 1 patient per 1,000 (1851). Omalizumab may increase the risk of cardiovascular events, thrombocytopenia or cancer (1852). Based on current data, omalizumab is not recommended for the treatment of CRS with nasal polyps (grade of recommendation: C). Mainly data on patients with previous sinus surgery are available.
6.7.4. Anti-IL-5
IL-5 is a key activator in eosinophil growth, recruitment and activation. High amounts of IL-5 were detected in polyp homogenisates, nasal secretions and blood serum of patients with NP. Mepolizumab (Glaxo Smith Kline) and reslizumab (Schering-Plough) are humanized anti–IL-5 mAb that reduce the number of eosinophils in blood and tissues (1853, 1854). Both antibodies currently undergo Phase II and III trials. In 2004, orphan designation (EU/3/04/213) was granted by the European Commission for mepolizumab for the treatment of hypereosinophilic syndrome. Two clinical trials with anti-IL-5 antibodies in CRS patients with nasal polyps were identified.
In a double-blind, placebo-controlled, randomized, 2-center phase I/II trial, 24 subjects with bilateral nasal polyps were randomized to receive a single intravenous infusion of reslizumab 3mg/kg or 1mg/kg or placebo (i.e. 8 patients per treatment arm). The post-injection observation period was 36 weeks. Adverse events did not significantly differ between treatment groups. No pharmacokinetic data and no detailed data on drop outs are provided. Main outcome measure for efficacy was an endoscopic nasal polyp score repeatedly evaluated for each nostril. Secondary efficacy parameters included peak nasal inspiratory airflow and nasal symptom scores. At no individual time point, a significant difference in the symptom scores or in the nasal peak inspiratory flow values was observed in both treatment groups compared with those in the placebo group. The total nasal polyp score was significantly decreased in the 1mg/kg group at week 12 compared with baseline values, however apparently not with the values of the control group. No dose response relation was observed. Blood eosinophil counts dropped significantly in both active groups, followed by a steep increase above baseline values 8-19 weeks post injection suggesting a rebound hypereosinophilia. Patients with nasal secretion IL-5 levels >40pg/ml were more likely to reveal a reduction of at least 1 polyp score on an 8 point scale when treated with anti-IL-5 (931).
In a further randomized, double-blind, placebo-controlled study, CRS-patients with nasal polyps received 2 single intravenous injections (28 days apart) of 750 mg of mepolizumab (20 subjects) or placebo (10 subjects). Post-injection observation period was 48 weeks. The primary end point was the reduction in an endoscopic nasal polyp score on an 8 point scale at 8 weeks after the first dosing (4 weeks after the second dose). Secondary outcome parameter included sinus opacification in CT scans, peak nasal inspiratory flow and symptom scores. Last observation carried forward imputation was used to handle missing data. Number and severity of adverse events did not differ between treatment groups. In the treatment group, nasal polyp scores improved 1.30±1.72 (SD) score points while it remained unchanged (0.00±0.94) in the control group, resulting in a treatment difference of 1.30±1.51 score points (p=0.028, Mann-Whitney U test). Moreover, significantly less sinus opacification was observed in the treatment arm (932).
The results of these clinical trials suggest that anti-IL-5 antibodies could play a role in the treatment of selected CRS-patients with nasal polyps. In a recent reslizumab study in asthma patients, nasopharyngitis, fatigue, and pharyngolaryngeal pain were common adverse events (1855). No data on patients without previous sinus surgery are available.

6.7.5. Antihistamines
One randomized placebo-controlled trial on antihistamines in CRS-patients with nasal polyps was identified. Forty-five surgically treated patients with residual or recurrent nasal polyps received either cetirizine 20mg b.i.d. (n=23) or placebo (n=22) for three months. Inhaled steroids for asthma treatment up to 800 µg per day were allowed as concomitant medication. Endoscopic polyp size, nasal symptom score at follow up visits and a nasal symptom daily diary cards served as outcome parameters. No primary study endpoint was defined and no power calculations are provided. In each group, 18 patients finished the study regularly, an IT analysis was performed. The method of missing value handling is not reported. Adverse events were equally distributed among treatment arms. The number and size of polyps remained unchanged during the study period. Nasal symptom scores at follow up visits did not significantly differ between the two treatment arms. In the daily diary cards, significantly less days with a symptom score <= 1 were observed for nasal hypersecretion (weeks 1 to 4, 5 to 8 and 9 to 12), sneezing (weeks 1 to 4 and 5 to 8) and nasal obstruction (weeks 9 to 12). However, daily dairy scores above >1 were rare for nasal hypersecretion and sneezing in the whole study population. No adjustment for multiple testing is reported (1856). Cetirizine is a safe antihistamine. Adverse effects include drowsiness; dry mouth and tiredness. Based on current data, cetirizine is not recommended for the treatment of CRS with nasal polyps (grade of recommendation: D). In patients with concomitant nasal allergies, antihistamines may be indicated. (grade of recommendation: C) No data on patients without previous sinus surgery are available.
6.7.6. Antimycotics
Eosinophilic rhinosinusitis is a non-invasive, chronic eosinophilic sinus inflammation frequently associated with nasal polyps. Viscid sinus secretions with eosinophil decay products, termed eosinophilic mucus by Bent and Kuhn (721), are characteristic for this condition. If fungal elements are detected by histology, fungal culture or molecular methods, the term eosinophilic fungal rhinosinusitis is appropriate. Eosinophilic fungal rhinosinusitis may be further divided in allergic fungal rhinosinusitis (AFRS) with a positive diagnostic test for IgE mediated allergy to the fungal elements detected within the sinus. It is considered an IgE mediated mucosal hypersensitivity directed against fungal antigens deposited on sinus mucosa (1857). If Type I allergy tests to moulds are negative, but eosinophilic mucus with fungal elements is found, the term non-allergic fungal eosinophilic rhinosinusitis is used (2069). Eosinophilic mucus may also occur in the absence of fungal elements and is categorized as non-fungal eosinophilic mucus rhinosinusitis.
Based on fungal detection and the presence of allergic mucus in almost all patients with chronic rhinosinusitis, Ponikau and coauthors proposed that CRS is generally caused by a dysregulated, but IgE independent immune response to fungal elements present on the mucosal surface (592, 702). As a consequence, reduction of fungus load should influence disease severity in all subtypes of CRS. This hypothesis led to a series of investigations, which did rather serve to proof this concept than to treat fungal disease. In these studies, patients complying with the AAO-HNS or EPOS definitions of CRS were included (8, 1205), irrespective of the presence of eosinophilic mucus and/or fungus detection.
6.7.6.1. Topical amphotericin B
In most trials with antifungals in CRS, amphotericin B was applied topically, either as a nasal spray or as a nasal irrigation. The majority of patients included in these trials suffered from CRS with polyps. However, not in all trials, the presence of nasal polyps was explicitly reported. Topical amphotericin B trials were extensively discussed in the last EPOS version. Since then, topical amphotericin B treatment was reviewed in 2 review articles and 1 Cochrane analysis. The authors conclude that the use of topical amphotericin B in patients with CRS with polyps is not justified (1585, 1858, 1859).
Amphotericin B is not systemically available after oral intake. Adverse events after topical nasal application include local irritation and rarely unpleasant smell sensations. Based on current data, topical amphotericin B is not recommended for the treatment of CRS with nasal polyps (grade of recommendation: A).
One report on topical nasal treatment with another antifungal was identified. In an uncontrolled study, 16 patients with previously treated allergic fungal sinusitis and worsening clinical symptoms received nasal fluconazole spray in addition to systemic steroids and/or systemic itraconazole. Stable disease or improvement was observed in 12/16 patients (1860).
6.7.6.2. Systemic antifungal treatments
There is 1 controlled study and few reports of uncontrolled studies of postoperative systemic antifungal treatment in patients with confirmed fungal rhinosinusitis.
Kennedy and co-authors prospectively compared oral terbinafine with placebo in fungus positive and fungus negative CRS patients. Treatment with terbinafine failed to improve symptoms or radiographic appearance of chronic rhinosinusitis even when nasal irrigation samples were positive for fungus on culture (1861).
Seiberling and co-authors performed a retrospective chart review of 23 patients with AFRS and non-allergic eosinophilic fungal sinusitis, who had failed maximal medical and surgical therapy. Patients with recurrent disease received itraconazole at a dose of 100mg b.i.d. for a minimum period of 6 months. Three patients had to stop treatment due to hepatic side effects, 4 patients did not respond and 16 patients showed a varying degree of symptom improvement including a decrease in the use of oral steroids and fungal mucus/polyps on endoscopy (1584). Rains and Mineck performed a chart review of 139 patients with allergic fungal rhinosinusitis and reported a benefit of highdose systemic itraconazole treatment in patients with recurrent disease (1862). Chan and co-authors treated 32 patients with fungal eosinophilic chronic rhinosinusitis who did not respond to surgery, oral cortisone and nasal amphotericin B spray with oral itraconazole for at least 3 months. Twelve patients has improved endoscopic findings following treatment, 15 showed no difference, and 5 were worse. Serum total IgE levels were not affected (1863). Rupa and co-workers treated 12 patients with fungus positive eosinophilic rhinosinusitis following sinus surgery with nasal steroids and oral itraconazole 200 mg daily for 12 weeks. All patients but one patient relapsed within the study period (1572).
Long-term itraconazole treatment has considerable adverse effects including nausea and fatigue. The main problem is hepatic toxicity with increased serum alanine tranferase levels in 4% of patients. Congestive heart failure is an infrequent side effect of itraconazole treatment. Itraconazole interacts with various other drugs. Drug interactions may increase the risk of congestive heart failure.
Based on current data, systemic antifungal treatment is not recommended in CRS with nasal polyps (grade of recommendation A).
One randomized placebo-controlled trial on antihistamines in CRS-patients with nasal polyps was identified. Forty-five surgically treated patients with residual or recurrent nasal polyps received either cetirizine 20mg b.i.d. (n=23) or placebo (n=22) for three months. Inhaled steroids for asthma treatment up to 800 µg per day were allowed as concomitant medication. Endoscopic polyp size, nasal symptom score at follow up visits and a nasal symptom daily diary cards served as outcome parameters. No primary study endpoint was defined and no power calculations are provided. In each group, 18 patients finished the study regularly, an IT analysis was performed. The method of missing value handling is not reported. Adverse events were equally distributed among treatment arms. The number and size of polyps remained unchanged during the study period. Nasal symptom scores at follow up visits did not significantly differ between the two treatment arms. In the daily diary cards, significantly less days with a symptom score <= 1 were observed for nasal hypersecretion (weeks 1 to 4, 5 to 8 and 9 to 12), sneezing (weeks 1 to 4 and 5 to 8) and nasal obstruction (weeks 9 to 12). However, daily dairy scores above >1 were rare for nasal hypersecretion and sneezing in the whole study population. No adjustment for multiple testing is reported (1856). Cetirizine is a safe antihistamine. Adverse effects include drowsiness; dry mouth and tiredness. Based on current data, cetirizine is not recommended for the treatment of CRS with nasal polyps (grade of recommendation: D). In patients with concomitant nasal allergies, antihistamines may be indicated. (grade of recommendation: C) No data on patients without previous sinus surgery are available.
6.7.6. Antimycotics
Eosinophilic rhinosinusitis is a non-invasive, chronic eosinophilic sinus inflammation frequently associated with nasal polyps. Viscid sinus secretions with eosinophil decay products, termed eosinophilic mucus by Bent and Kuhn (721), are characteristic for this condition. If fungal elements are detected by histology, fungal culture or molecular methods, the term eosinophilic fungal rhinosinusitis is appropriate. Eosinophilic fungal rhinosinusitis may be further divided in allergic fungal rhinosinusitis (AFRS) with a positive diagnostic test for IgE mediated allergy to the fungal elements detected within the sinus. It is considered an IgE mediated mucosal hypersensitivity directed against fungal antigens deposited on sinus mucosa (1857). If Type I allergy tests to moulds are negative, but eosinophilic mucus with fungal elements is found, the term non-allergic fungal eosinophilic rhinosinusitis is used (2069). Eosinophilic mucus may also occur in the absence of fungal elements and is categorized as non-fungal eosinophilic mucus rhinosinusitis.
Based on fungal detection and the presence of allergic mucus in almost all patients with chronic rhinosinusitis, Ponikau and coauthors proposed that CRS is generally caused by a dysregulated, but IgE independent immune response to fungal elements present on the mucosal surface (592, 702). As a consequence, reduction of fungus load should influence disease severity in all subtypes of CRS. This hypothesis led to a series of investigations, which did rather serve to proof this concept than to treat fungal disease. In these studies, patients complying with the AAO-HNS or EPOS definitions of CRS were included (8, 1205), irrespective of the presence of eosinophilic mucus and/or fungus detection.
6.7.6.1. Topical amphotericin B
In most trials with antifungals in CRS, amphotericin B was applied topically, either as a nasal spray or as a nasal irrigation. The majority of patients included in these trials suffered from CRS with polyps. However, not in all trials, the presence of nasal polyps was explicitly reported. Topical amphotericin B trials were extensively discussed in the last EPOS version. Since then, topical amphotericin B treatment was reviewed in 2 review articles and 1 Cochrane analysis. The authors conclude that the use of topical amphotericin B in patients with CRS with polyps is not justified (1585, 1858, 1859).
Amphotericin B is not systemically available after oral intake. Adverse events after topical nasal application include local irritation and rarely unpleasant smell sensations. Based on current data, topical amphotericin B is not recommended for the treatment of CRS with nasal polyps (grade of recommendation: A).
One report on topical nasal treatment with another antifungal was identified. In an uncontrolled study, 16 patients with previously treated allergic fungal sinusitis and worsening clinical symptoms received nasal fluconazole spray in addition to systemic steroids and/or systemic itraconazole. Stable disease or improvement was observed in 12/16 patients (1860).
6.7.6.2. Systemic antifungal treatments
There is 1 controlled study and few reports of uncontrolled studies of postoperative systemic antifungal treatment in patients with confirmed fungal rhinosinusitis.
Kennedy and co-authors prospectively compared oral terbinafine with placebo in fungus positive and fungus negative CRS patients. Treatment with terbinafine failed to improve symptoms or radiographic appearance of chronic rhinosinusitis even when nasal irrigation samples were positive for fungus on culture (1861).
Seiberling and co-authors performed a retrospective chart review of 23 patients with AFRS and non-allergic eosinophilic fungal sinusitis, who had failed maximal medical and surgical therapy. Patients with recurrent disease received itraconazole at a dose of 100mg b.i.d. for a minimum period of 6 months. Three patients had to stop treatment due to hepatic side effects, 4 patients did not respond and 16 patients showed a varying degree of symptom improvement including a decrease in the use of oral steroids and fungal mucus/polyps on endoscopy (1584). Rains and Mineck performed a chart review of 139 patients with allergic fungal rhinosinusitis and reported a benefit of highdose systemic itraconazole treatment in patients with recurrent disease (1862). Chan and co-authors treated 32 patients with fungal eosinophilic chronic rhinosinusitis who did not respond to surgery, oral cortisone and nasal amphotericin B spray with oral itraconazole for at least 3 months. Twelve patients has improved endoscopic findings following treatment, 15 showed no difference, and 5 were worse. Serum total IgE levels were not affected (1863). Rupa and co-workers treated 12 patients with fungus positive eosinophilic rhinosinusitis following sinus surgery with nasal steroids and oral itraconazole 200 mg daily for 12 weeks. All patients but one patient relapsed within the study period (1572).
Long-term itraconazole treatment has considerable adverse effects including nausea and fatigue. The main problem is hepatic toxicity with increased serum alanine tranferase levels in 4% of patients. Congestive heart failure is an infrequent side effect of itraconazole treatment. Itraconazole interacts with various other drugs. Drug interactions may increase the risk of congestive heart failure.
Based on current data, systemic antifungal treatment is not recommended in CRS with nasal polyps (grade of recommendation A).
6.7.7. Furosemide
Aerosolized furosemide was used in the treatment of acute asthma attacks (1864). Several mechanisms including induction of relaxant prostaglandins, blockade of mediator production from inflammatory cells, and regulation of ion exchange in the airway epithelium were proposed to explain its anti-asthmatic activity (1865).
In a controlled, open label study, 64 CRS-patients with nasal polyps received furosemide nasal spray (200 µg daily) following endonasal sinus surgery. A control group of 40 patients received no treatment. The mode of randomization is not detailed. After 6 years, 4 patients in the furosemide group and 12 patients in the control group had experienced recurrent disease (p<0.01). Drop out handling is not detailed in the report (1866). In 2003, Passali and co-workers published long term results of topical furosemide treatment in 170 CRS patients with nasal polyps following endonasal surgery. From 1991 to 1997, patients were randomly assigned to furosemide or control treatment. It appears that patients included in the 2000 report were included also in this evaluation. Furosemide was 1:1 diluted 2 mL isotonic sodium chloride solution administered as nasal spray (100µg per nostril and day). One-month treatment alternated with 1 month without treatment. The intervals without treatment were then gradually extended. In the control group, no specific treatment was given. In 1998, control treatment was stopped and mometasone was given instead. Patients received 2 puffs mometasone spray per day per nostril with the same monthly interruptions used in the furosemide group. No adverse events were registered. Seventeen (17.5%) of 97 patients in the furosemide group, 12 (30.0%) of 40 patients in the control group, and 8 (24.2%) of 33 patients in the mometasone group experienced nasal polyposis relapses (p>0.2) (1807).
Mode of randomization, number of screened patients, drop out rate and missing data handling are not reported. As a consequence, the value of these two trials is difficult to interpret. In a randomised trial, 40 CRS-patients with nasal polyps were randomly allocated to treatment either with oral methylprednisolone (1 mg/kg/day) or inhalation of 6.6 mmol/l furosemide solution/10 min through a jet nebulizer (20 mg/ day furosemide) for 7 days prior to endonasal sinus surgery. The mode of randomization is not detailed. Twelve patients had undergone previous sinus surgeries ('recurrences'). Study endpoints were a nasal symptom score and an endoscopic polyp score assessed before and after treatment. Serum potassium levels and blood pressure were monitored before and during 1 h after each inhalation in the furosemide group. No systemic diuretic effects were observed. Total symptom scores changed from 15.50±3.44 (mean ± SD) to 9.55±3.55 in the methylprednisolone group and from 15.60±3.91 to 9.80±3.69 in the furosemide group (both p<0.01). Nasal polyp scores changed from 2.38±0.67 to 1.95±0.78 in the methylprednisolone group and from 2.23±0.89 to 1.68±0.89 in the furosemide group (both p<0.01) (1867).
The bioavailability of furosemide after oral intake is approximately 60%. Data on nasal uptake are not available. Main side effects of oral furosemide are water and electrolyte imbalances. Based on current data, (postoperative) long-term nasal furosemide treatment is not recommended (grade of recommendation D). Further studies are needed to assess the possible benefit of preoperative short term, high dose nasal furosemide treatment.
6.7.8. Immunosuppressants
In glucocorticoid-dependent asthma, immunosuppressants including methotrexate may aid to reduce the steroid dose (1868). There are two anecdotal reports that nasal polyps may substantially improve, if methotrexate is given in steroiddependent asthma or malignant conditions with concomitant nasal polyps (2070,2071). Based on current data, the use of immunosuppressants is not recommended in CRS with nasal polyps (grade of recommendation D).
6.7.9. Leukotriene antagonists
Ragab and co-authors evaluated the efficacy and tolerability of montelukast as an add-on therapy in the treatment of nasal polyposis in association with asthma. In 44 adult CRSpatients with nasal polyposis (24 with AERD) refractory to medical therapy with long-term intranasal corticosteroids; oral montelukast (10 mg/day) was given for three months as an add-on therapy to intranasal and inhaled corticosteroids. Main outcome parameter was a clinical score based on the results of clinical symptoms, examination, acoustic rhinometry, and peak nasal inspiratory flow. The majority of patients experienced clinical improvement. Improvement of nasal polyp score was significant, irrespective if the patients suffered from AERD or not (1515).
In a single-blind, randomized, placebo-controlled cross over trial, 24 patients with asthma and post-FESS CRS with nasal polyps were enrolled. One group started with a 4-week placebo phase and continued with 6 weeks of montelukast treatment (10 mg/day) while the other group started with montelukast treatment for 6 weeks and continued with placebo for 4 weeks. Statistical analysis did not account for the special problems of cross-over designs and the data presentation is not appropriate for this type of trial design. During montelukast treatment, the mean scores decreased from 1.8 to 0.6 for nasal blockage, from 1.5 to 0.6 for rhinorrhoea, and from 0.6 to 0.25 for itching. In a similar manner, the quality of smell improved from 2.0 to 0.3 and the total symptom score improved from 5.9 to 1.75 (p<.001). No significant changes in symptoms were observed during the placebo period. Significant improvements were also noted in nasal endoscopy scores. Various proinflammatory biomarkers in nasal lavages revealed significant improvements (1869). In an uncontrolled, open label study 26 CRS-patients with nasal polyps without AERD received 10 mg montelukast daily for 3 months on top on long-term nasal steroid therapy. Symptom scores were assessed before and after the 3-month treatment interval in 24 patients, who finished the study regularly. The symptoms improved in 17 patients (71%) and remained the same or worsened in 7 patients (29%). Patients with concomitant nasal allergy responded better than patients without allergy (1870).
In an uncontrolled open label study, 26 patients with nasal polyps received oral zafirlukast 10 mg bid or zileuton 600 mg qid on top of systemic steroid therapy. Nasal symptom scores were assessed before and after a treatment period of 7 months. Concomitant asthma was present in 14 patients, 2 patients suffered from AERD. Overall, 26 of the 36 patients (72%) experienced improvement in their symptomatology after starting antileukotriene therapy. No patient experienced a worsening of symptoms. The remaining 10 patients (28%) experienced no change (1871). In an uncontrolled open label study, 20 patients with nasal polyps and bronchial asthma received oral montelukast 10 mg/ day on top of nasal and inhaled steroid therapy for 1 year. Study endpoints included nasal polyp scores and sinus opacification on pre- and post treatment CT scans. Nasal allergy was present in 11 patients and 8 were judged to suffer from AERD on the basis of their history. Nasal polyp and CT scores improved significantly during treatment (1872).
Following endoscopic sinus surgery, Mostafa and coworkers randomly assigned 40 patients with nasal polyps without asthma either to 10 mg montelukast daily or 400 µg beclomethasone nasal spray daily for 1 year. Study endpoints included disease relapse and nasal symptom scores. No differences in disease relapse frequency were noted. Nasal beclomethasone was superior to montelukast controlling nasal obstruction, rhinorrhoea, sense of smell and sneezing. The onset of montelukast action was prolonged, with the maximum therapeutic effect seen after 6 months of treatment (1873). In a randomized, placebo-controlled trial, 20 patients with nasal polyps were treated with montelukast 10 mg/day and 10 patients received placebo treatment for 4 weeks. Nasal polyp scores, eosinophila cationic protein levels in nasal secretions and HRQL employing a modified Juniper score were assessed before and after treatment. No significant changes in polyp scores and nasal ECP levels were observed. In some HRQL parameters, better scores were observed in the treatment group (1874) In a randomized, controlled trial without allocation concealment, 38 consecutive adult patients with bilateral nasal polyps were treated with oral prednisolone for 14 days and budenoside nasal spray for 8 weeks (n=18). Twenty subjects received similar treatment with additional oral montelukast 10 mg/day for 8 weeks. Concomitant nasal allergy was more frequent among montelukast treated patients. Outcome measures included nasal symptom scores and the SF-36 HRQL questionnaire. When compared with subjects treated with steroid alone, subjects treated with montelukast showed a significant reduction in symptom scores at eight weeks with respect to headache, facial pain, and sneezing. However, montelukast therapy did not have a significant effect on the overall symptom score or on symptoms of nasal blockage, hyposmia, or nasal discharge (1875).
Adverse effects of leukotriene antagonists include skin rash, mood or behavior changes, tremors or shaking and occasional worsening of sinus symptoms and asthma. Current data do not support anti-leukotriene therapy in CRS-patients with polyps. Leukotriene antagonists are not recommended for the treatment of CRSwNP (Grade of recommendation A).
Aerosolized furosemide was used in the treatment of acute asthma attacks (1864). Several mechanisms including induction of relaxant prostaglandins, blockade of mediator production from inflammatory cells, and regulation of ion exchange in the airway epithelium were proposed to explain its anti-asthmatic activity (1865).
In a controlled, open label study, 64 CRS-patients with nasal polyps received furosemide nasal spray (200 µg daily) following endonasal sinus surgery. A control group of 40 patients received no treatment. The mode of randomization is not detailed. After 6 years, 4 patients in the furosemide group and 12 patients in the control group had experienced recurrent disease (p<0.01). Drop out handling is not detailed in the report (1866). In 2003, Passali and co-workers published long term results of topical furosemide treatment in 170 CRS patients with nasal polyps following endonasal surgery. From 1991 to 1997, patients were randomly assigned to furosemide or control treatment. It appears that patients included in the 2000 report were included also in this evaluation. Furosemide was 1:1 diluted 2 mL isotonic sodium chloride solution administered as nasal spray (100µg per nostril and day). One-month treatment alternated with 1 month without treatment. The intervals without treatment were then gradually extended. In the control group, no specific treatment was given. In 1998, control treatment was stopped and mometasone was given instead. Patients received 2 puffs mometasone spray per day per nostril with the same monthly interruptions used in the furosemide group. No adverse events were registered. Seventeen (17.5%) of 97 patients in the furosemide group, 12 (30.0%) of 40 patients in the control group, and 8 (24.2%) of 33 patients in the mometasone group experienced nasal polyposis relapses (p>0.2) (1807).
Mode of randomization, number of screened patients, drop out rate and missing data handling are not reported. As a consequence, the value of these two trials is difficult to interpret. In a randomised trial, 40 CRS-patients with nasal polyps were randomly allocated to treatment either with oral methylprednisolone (1 mg/kg/day) or inhalation of 6.6 mmol/l furosemide solution/10 min through a jet nebulizer (20 mg/ day furosemide) for 7 days prior to endonasal sinus surgery. The mode of randomization is not detailed. Twelve patients had undergone previous sinus surgeries ('recurrences'). Study endpoints were a nasal symptom score and an endoscopic polyp score assessed before and after treatment. Serum potassium levels and blood pressure were monitored before and during 1 h after each inhalation in the furosemide group. No systemic diuretic effects were observed. Total symptom scores changed from 15.50±3.44 (mean ± SD) to 9.55±3.55 in the methylprednisolone group and from 15.60±3.91 to 9.80±3.69 in the furosemide group (both p<0.01). Nasal polyp scores changed from 2.38±0.67 to 1.95±0.78 in the methylprednisolone group and from 2.23±0.89 to 1.68±0.89 in the furosemide group (both p<0.01) (1867).
The bioavailability of furosemide after oral intake is approximately 60%. Data on nasal uptake are not available. Main side effects of oral furosemide are water and electrolyte imbalances. Based on current data, (postoperative) long-term nasal furosemide treatment is not recommended (grade of recommendation D). Further studies are needed to assess the possible benefit of preoperative short term, high dose nasal furosemide treatment.
6.7.8. Immunosuppressants
In glucocorticoid-dependent asthma, immunosuppressants including methotrexate may aid to reduce the steroid dose (1868). There are two anecdotal reports that nasal polyps may substantially improve, if methotrexate is given in steroiddependent asthma or malignant conditions with concomitant nasal polyps (2070,2071). Based on current data, the use of immunosuppressants is not recommended in CRS with nasal polyps (grade of recommendation D).
6.7.9. Leukotriene antagonists
Ragab and co-authors evaluated the efficacy and tolerability of montelukast as an add-on therapy in the treatment of nasal polyposis in association with asthma. In 44 adult CRSpatients with nasal polyposis (24 with AERD) refractory to medical therapy with long-term intranasal corticosteroids; oral montelukast (10 mg/day) was given for three months as an add-on therapy to intranasal and inhaled corticosteroids. Main outcome parameter was a clinical score based on the results of clinical symptoms, examination, acoustic rhinometry, and peak nasal inspiratory flow. The majority of patients experienced clinical improvement. Improvement of nasal polyp score was significant, irrespective if the patients suffered from AERD or not (1515).
In a single-blind, randomized, placebo-controlled cross over trial, 24 patients with asthma and post-FESS CRS with nasal polyps were enrolled. One group started with a 4-week placebo phase and continued with 6 weeks of montelukast treatment (10 mg/day) while the other group started with montelukast treatment for 6 weeks and continued with placebo for 4 weeks. Statistical analysis did not account for the special problems of cross-over designs and the data presentation is not appropriate for this type of trial design. During montelukast treatment, the mean scores decreased from 1.8 to 0.6 for nasal blockage, from 1.5 to 0.6 for rhinorrhoea, and from 0.6 to 0.25 for itching. In a similar manner, the quality of smell improved from 2.0 to 0.3 and the total symptom score improved from 5.9 to 1.75 (p<.001). No significant changes in symptoms were observed during the placebo period. Significant improvements were also noted in nasal endoscopy scores. Various proinflammatory biomarkers in nasal lavages revealed significant improvements (1869). In an uncontrolled, open label study 26 CRS-patients with nasal polyps without AERD received 10 mg montelukast daily for 3 months on top on long-term nasal steroid therapy. Symptom scores were assessed before and after the 3-month treatment interval in 24 patients, who finished the study regularly. The symptoms improved in 17 patients (71%) and remained the same or worsened in 7 patients (29%). Patients with concomitant nasal allergy responded better than patients without allergy (1870).
In an uncontrolled open label study, 26 patients with nasal polyps received oral zafirlukast 10 mg bid or zileuton 600 mg qid on top of systemic steroid therapy. Nasal symptom scores were assessed before and after a treatment period of 7 months. Concomitant asthma was present in 14 patients, 2 patients suffered from AERD. Overall, 26 of the 36 patients (72%) experienced improvement in their symptomatology after starting antileukotriene therapy. No patient experienced a worsening of symptoms. The remaining 10 patients (28%) experienced no change (1871). In an uncontrolled open label study, 20 patients with nasal polyps and bronchial asthma received oral montelukast 10 mg/ day on top of nasal and inhaled steroid therapy for 1 year. Study endpoints included nasal polyp scores and sinus opacification on pre- and post treatment CT scans. Nasal allergy was present in 11 patients and 8 were judged to suffer from AERD on the basis of their history. Nasal polyp and CT scores improved significantly during treatment (1872).
Following endoscopic sinus surgery, Mostafa and coworkers randomly assigned 40 patients with nasal polyps without asthma either to 10 mg montelukast daily or 400 µg beclomethasone nasal spray daily for 1 year. Study endpoints included disease relapse and nasal symptom scores. No differences in disease relapse frequency were noted. Nasal beclomethasone was superior to montelukast controlling nasal obstruction, rhinorrhoea, sense of smell and sneezing. The onset of montelukast action was prolonged, with the maximum therapeutic effect seen after 6 months of treatment (1873). In a randomized, placebo-controlled trial, 20 patients with nasal polyps were treated with montelukast 10 mg/day and 10 patients received placebo treatment for 4 weeks. Nasal polyp scores, eosinophila cationic protein levels in nasal secretions and HRQL employing a modified Juniper score were assessed before and after treatment. No significant changes in polyp scores and nasal ECP levels were observed. In some HRQL parameters, better scores were observed in the treatment group (1874) In a randomized, controlled trial without allocation concealment, 38 consecutive adult patients with bilateral nasal polyps were treated with oral prednisolone for 14 days and budenoside nasal spray for 8 weeks (n=18). Twenty subjects received similar treatment with additional oral montelukast 10 mg/day for 8 weeks. Concomitant nasal allergy was more frequent among montelukast treated patients. Outcome measures included nasal symptom scores and the SF-36 HRQL questionnaire. When compared with subjects treated with steroid alone, subjects treated with montelukast showed a significant reduction in symptom scores at eight weeks with respect to headache, facial pain, and sneezing. However, montelukast therapy did not have a significant effect on the overall symptom score or on symptoms of nasal blockage, hyposmia, or nasal discharge (1875).
Adverse effects of leukotriene antagonists include skin rash, mood or behavior changes, tremors or shaking and occasional worsening of sinus symptoms and asthma. Current data do not support anti-leukotriene therapy in CRS-patients with polyps. Leukotriene antagonists are not recommended for the treatment of CRSwNP (Grade of recommendation A).
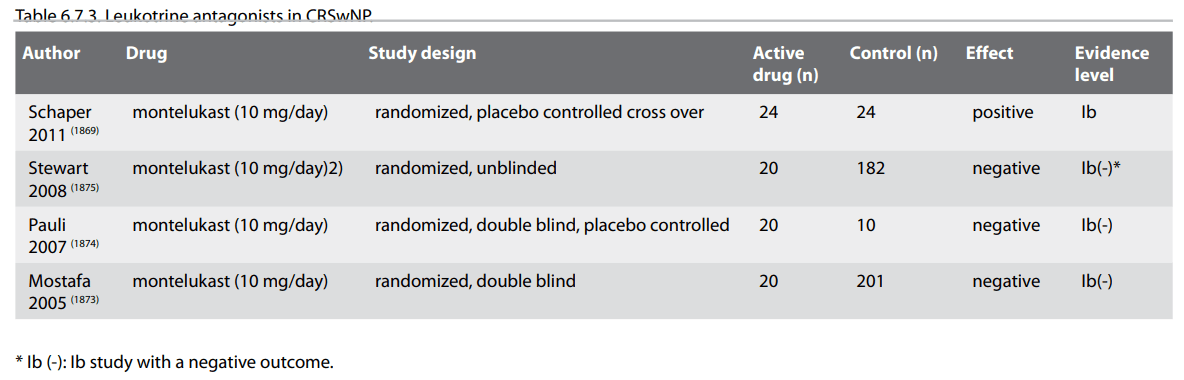
6.7.10. Aspirin desensitisation
Aspirin-exacerbated respiratory disease (AERD) is characterized by chronic rhinosinusitis with nasal polyps, bronchial asthma and hypersensitivity to inhibitors of cyclooxygenase-1 (Cox-1) including aspirin and other non-steroidal anti-inflammatory drugs (1525). The diagnosis is mainly based on patient history and aspirin provocation tests (1506). In AERD patients, aspirin may induce a period lasting 24 to 72 hours, in which patients are refractory to repeated aspirin challenges and experience clinical improvement (1876). Based on this observation, several oral and nasal aspirin desensitisation protocols were developed. Most widely used is the Scripps-clinics oral aspirin desensitisation protocol, in which, following a stepwise dose increase, 625 mg aspirin is orally administered twice daily (1525). Several case series suggest albeit weak clinical benefit from oral aspirin desensitisation (1526, 1877), but no randomized placebocontrolled trial on oral aspirin desensitisation in patients with AERD could be identified. In a cross-over trial, 25 AERD patients were treated with oral aspirin in 3 different dosages or with placebo for 3 months, separated by a 1 month wash out phase. Symptom scores and concomitant medication use in the two trial phases were compared with 1-sided t-test. The mode of randomization is not detailed in the publication. In the aspirin phase, less nasal symptoms and less nasal steroid use was observed. Lower respiratory tract symptoms, values of FEV1, and the use of anti-asthmatic medications including prednisone were not better during ASA treatment (1825).
In one clinical trial, 14 patients who reacted positively in an aspirin provocation test were alternately allocated to take 100 mg aspirin or 300 mg aspirin daily and were followed for at least 1 year. After 1 year of aspirin therapy, all patients of the 100mg group (100%; 95%CI, 59–100%) had developed recurrent nasal polyps. No patient of the 300mg group showed recurrent nasal polyps in endoscopic examination (0%; 95% CI, 0–41%) (1878). Nasal administration of lysine-aspirin reduces the risk of severe hypersensitivity reactions and the frequency of gastrointestinal side effects associated with oral aspirin desensitisation. Some retrospective studies reported clinical benefit from nasal lysine-aspirin treatment (1824, 1529). In a randomized, double-blind placebo-controlled cross over trial, AERD patients with positive nasal lysine-aspirin challenge received either 16mg nasal lysine aspirin or placebo every 48 hrs. for 6 months. Of 22 patients entering the trial, 11 were eligible for analysis. Multivariate analysis of measured parameters did not reveal a significant clinical benefit to patients receiving topical lysine-aspirin compared with placebo (1528).
Oral aspirin desensitisation is associated with the risk of severe hypersensitivity reactions and gastrointestinal side effects. Based on current data, the benefit of oral or nasal aspirin desensitisation in patients AERD remains elusive. Aspirin desensitisation is currently not recommended outside clinical trials (grade of recommendation D).
6.7.11. Capsaicin
Two case series and 1 RCT on nasal capsaicin treatment following sinus surgery were identified (1879-1881). In the RCT, 29 patients capsaicin soaked cotton pellets were brought into the middle meatus of both nostrils for 20 min once a week for 5 weeks. An age and gender matched control group of 22 patients were treated with the capsaicin vehicle alone (70% ethanol). Nasal symptom scores and a nasal endoscopy score were the main outcome parameters. Patients treated with capsaicin showed a significant smaller staging of their nasal polyposis compared with the control group (p<0.001) (1879) (grade of recommendation: C).
6.7.12. Various other medical treatments
Single studies and anecdotal reports on various topical and systemic treatments do not allow to recommend their use in CRSwNP including nasal decongestants (1882), mucolytics (1883), postoperative saline douches (1738) or spray (1884), manuka honey (1587), proton pump inhibitors or phytopreparations (no data available for CRSwNP).
Aspirin-exacerbated respiratory disease (AERD) is characterized by chronic rhinosinusitis with nasal polyps, bronchial asthma and hypersensitivity to inhibitors of cyclooxygenase-1 (Cox-1) including aspirin and other non-steroidal anti-inflammatory drugs (1525). The diagnosis is mainly based on patient history and aspirin provocation tests (1506). In AERD patients, aspirin may induce a period lasting 24 to 72 hours, in which patients are refractory to repeated aspirin challenges and experience clinical improvement (1876). Based on this observation, several oral and nasal aspirin desensitisation protocols were developed. Most widely used is the Scripps-clinics oral aspirin desensitisation protocol, in which, following a stepwise dose increase, 625 mg aspirin is orally administered twice daily (1525). Several case series suggest albeit weak clinical benefit from oral aspirin desensitisation (1526, 1877), but no randomized placebocontrolled trial on oral aspirin desensitisation in patients with AERD could be identified. In a cross-over trial, 25 AERD patients were treated with oral aspirin in 3 different dosages or with placebo for 3 months, separated by a 1 month wash out phase. Symptom scores and concomitant medication use in the two trial phases were compared with 1-sided t-test. The mode of randomization is not detailed in the publication. In the aspirin phase, less nasal symptoms and less nasal steroid use was observed. Lower respiratory tract symptoms, values of FEV1, and the use of anti-asthmatic medications including prednisone were not better during ASA treatment (1825).
In one clinical trial, 14 patients who reacted positively in an aspirin provocation test were alternately allocated to take 100 mg aspirin or 300 mg aspirin daily and were followed for at least 1 year. After 1 year of aspirin therapy, all patients of the 100mg group (100%; 95%CI, 59–100%) had developed recurrent nasal polyps. No patient of the 300mg group showed recurrent nasal polyps in endoscopic examination (0%; 95% CI, 0–41%) (1878). Nasal administration of lysine-aspirin reduces the risk of severe hypersensitivity reactions and the frequency of gastrointestinal side effects associated with oral aspirin desensitisation. Some retrospective studies reported clinical benefit from nasal lysine-aspirin treatment (1824, 1529). In a randomized, double-blind placebo-controlled cross over trial, AERD patients with positive nasal lysine-aspirin challenge received either 16mg nasal lysine aspirin or placebo every 48 hrs. for 6 months. Of 22 patients entering the trial, 11 were eligible for analysis. Multivariate analysis of measured parameters did not reveal a significant clinical benefit to patients receiving topical lysine-aspirin compared with placebo (1528).
Oral aspirin desensitisation is associated with the risk of severe hypersensitivity reactions and gastrointestinal side effects. Based on current data, the benefit of oral or nasal aspirin desensitisation in patients AERD remains elusive. Aspirin desensitisation is currently not recommended outside clinical trials (grade of recommendation D).
6.7.11. Capsaicin
Two case series and 1 RCT on nasal capsaicin treatment following sinus surgery were identified (1879-1881). In the RCT, 29 patients capsaicin soaked cotton pellets were brought into the middle meatus of both nostrils for 20 min once a week for 5 weeks. An age and gender matched control group of 22 patients were treated with the capsaicin vehicle alone (70% ethanol). Nasal symptom scores and a nasal endoscopy score were the main outcome parameters. Patients treated with capsaicin showed a significant smaller staging of their nasal polyposis compared with the control group (p<0.001) (1879) (grade of recommendation: C).
6.7.12. Various other medical treatments
Single studies and anecdotal reports on various topical and systemic treatments do not allow to recommend their use in CRSwNP including nasal decongestants (1882), mucolytics (1883), postoperative saline douches (1738) or spray (1884), manuka honey (1587), proton pump inhibitors or phytopreparations (no data available for CRSwNP).
6.8. Evidence based Surgery for CRSwNP
6.8.1. Introduction
Nasal polyps affect approximately 20% of patients with CRS. From a clinical, radiological and histological perspective the mucosal inflammatory response is more florid in CRS patients with nasal polyps than in those without, and the rate of relapse after surgery for nasal polyps tends to be higher (1885).
Surgical intervention in the treatment of nasal polyps is considered in patients who fail to improve after a trial of maximal medical treatment. Functional endoscopic sinus surgery involves the clearance of polyps and polypoid mucosa and opening of the sinus ostia. The removal of inflammatory tissue and reduction of the load of antigens inciting that inflammation, as well as the improvement of sinus ventilation and mucociliary clearance, are the probable mechanisms whereby FESS improves symptoms in nasal polyposis.
The optimal surgical management of nasal polyps has not yet been established. There are a number of factors contributing to the difficulty in gathering clinical data on which to base surgical management. A number of studies fail to distinguish between CRS with and without polyps. However in those studies that do, the distinction made on clinical grounds preoperatively (whether polyps are visible in the middle meatus) is itself imperfect. Many cases in which no polyps can be observed have impressively polypoid mucosa within the sinuses at the time of operation. There are very few RCT's which compare medical and surgical treatment with the extent of surgical resection required to optimize outcomes, hence this is largely unknown. Functional endoscopic sinus surgery describes an approach and not a standardized operation. The efficacy of procedures may well be dependent on their extent and so the specific details of the procedures performed need be considered carefully when assessing the reported efficacy.
The outcome post polyp surgery is influenced by whether the polyps are idiopathic or related to an underlying mucosal condition such as aspirin exacerbated respiratory disease, cystic fibrosis or primary ciliary dyskinaesia. However in both idiopathic and secondary nasal polyps, the long-term efficacy of surgery is almost certainly influenced by the regimen of medical treatment prescribed postoperatively and the subsequent compliance with this regimen.
In this chapter the evidence for efficacy of surgery will be reviewed, and compared to medical treatment alone. This is not an easy comparison to make as it is generally agreed that surgery is only indicated when medical therapy has failed. The issue of extent of surgery will be addressed, and the impact of underlying conditions and medical treatment have on surgical outcome will be summarized. Surgery for nasal polyps has been associated with a high rate of revision, and the role of second line procedures such as endoscopic modified Lothrop procedure will be discussed.
6.8.2. Efficacy of surgery for nasal polyposis
Endoscopic sinus surgery for nasal polyposis has been generally reported to be a safe and effective procedure.
A number of series have demonstrated that sinus surgery in patients with nasal polyps can result in a prolonged reduction of nasal symptoms and an improvement in quality of life. Dalziel et al. evaluated 33 articles published between 1978 and 2001 (1886). The review included three studies comparing FESS with Caldwell–Luc or other endonasal procedures (n=240), three nonrandomized studies comparing different surgical approaches (n=2,699) and 27 case series (n=8,208). Seven studies included only patients with polyps and 26 had CRS had with and without polyps. Patients judged their symptoms to be 'improved' or 'greatly improved' in 75 to 95% of cases. The percentage of overall complications was low (1.4% for FESS compared and 0.8% for traditional procedures). The implications of this review are that FESS is safe and effective treatment for the great majority of patients.
Two-thirds (2176) of the 3,128 patients included in the National Comparative Audit had CRS with nasal polyps (1757). In this prospective cohort study, a significant improvement in SNOT-22 scores was demonstrated at 3, 12 and 36 months with CRSwNP patients were found to benefit more from surgery than those with CRSsNP. Revision surgery was indicated in 3.6% of patients at 12 months and 11.8% at 36 months (1757).
6.8.3. Efficacy of surgery for nasal polyps compared to CRSsNP
The efficacy of FESS in patients with CRSwNP is at least as great as in patients with CRSsNP
There is some evidence that a significantly higher rate of recurrent surgery is required in patients with nasal polyposis than those without polyps (1887). Despite the increased rates of revision, patients with polyps may have more improvement following sinus surgery than CRSsNP patients (1757). In one large series, FESS was performed in 251 patients with medically refractory CRS (86 with polyps and 165 without), and the patients followed for at least 12 months. Symptom scores improved significantly in both groups (p<0.001). There were no significant differences between the groups except in oropharyngeal symptoms, which were improved more in the non-polyp patients (1888)..
In another series, 43 patients with polyps were compared with 76 patients without polyps before and after ESS. Mean follow-up was 1.5 years. Patients were analysed prospectively based on CT scans, endoscopy, quality-of-life (QOL) assessment and visual analog symptom scales. Despite significantly worse objective scores, patients with polyps surprisingly reported significantly better QOL scores and less facial pain or headache both pre- and postoperatively (1885).
6.8.1. Introduction
Nasal polyps affect approximately 20% of patients with CRS. From a clinical, radiological and histological perspective the mucosal inflammatory response is more florid in CRS patients with nasal polyps than in those without, and the rate of relapse after surgery for nasal polyps tends to be higher (1885).
Surgical intervention in the treatment of nasal polyps is considered in patients who fail to improve after a trial of maximal medical treatment. Functional endoscopic sinus surgery involves the clearance of polyps and polypoid mucosa and opening of the sinus ostia. The removal of inflammatory tissue and reduction of the load of antigens inciting that inflammation, as well as the improvement of sinus ventilation and mucociliary clearance, are the probable mechanisms whereby FESS improves symptoms in nasal polyposis.
The optimal surgical management of nasal polyps has not yet been established. There are a number of factors contributing to the difficulty in gathering clinical data on which to base surgical management. A number of studies fail to distinguish between CRS with and without polyps. However in those studies that do, the distinction made on clinical grounds preoperatively (whether polyps are visible in the middle meatus) is itself imperfect. Many cases in which no polyps can be observed have impressively polypoid mucosa within the sinuses at the time of operation. There are very few RCT's which compare medical and surgical treatment with the extent of surgical resection required to optimize outcomes, hence this is largely unknown. Functional endoscopic sinus surgery describes an approach and not a standardized operation. The efficacy of procedures may well be dependent on their extent and so the specific details of the procedures performed need be considered carefully when assessing the reported efficacy.
The outcome post polyp surgery is influenced by whether the polyps are idiopathic or related to an underlying mucosal condition such as aspirin exacerbated respiratory disease, cystic fibrosis or primary ciliary dyskinaesia. However in both idiopathic and secondary nasal polyps, the long-term efficacy of surgery is almost certainly influenced by the regimen of medical treatment prescribed postoperatively and the subsequent compliance with this regimen.
In this chapter the evidence for efficacy of surgery will be reviewed, and compared to medical treatment alone. This is not an easy comparison to make as it is generally agreed that surgery is only indicated when medical therapy has failed. The issue of extent of surgery will be addressed, and the impact of underlying conditions and medical treatment have on surgical outcome will be summarized. Surgery for nasal polyps has been associated with a high rate of revision, and the role of second line procedures such as endoscopic modified Lothrop procedure will be discussed.
6.8.2. Efficacy of surgery for nasal polyposis
Endoscopic sinus surgery for nasal polyposis has been generally reported to be a safe and effective procedure.
A number of series have demonstrated that sinus surgery in patients with nasal polyps can result in a prolonged reduction of nasal symptoms and an improvement in quality of life. Dalziel et al. evaluated 33 articles published between 1978 and 2001 (1886). The review included three studies comparing FESS with Caldwell–Luc or other endonasal procedures (n=240), three nonrandomized studies comparing different surgical approaches (n=2,699) and 27 case series (n=8,208). Seven studies included only patients with polyps and 26 had CRS had with and without polyps. Patients judged their symptoms to be 'improved' or 'greatly improved' in 75 to 95% of cases. The percentage of overall complications was low (1.4% for FESS compared and 0.8% for traditional procedures). The implications of this review are that FESS is safe and effective treatment for the great majority of patients.
Two-thirds (2176) of the 3,128 patients included in the National Comparative Audit had CRS with nasal polyps (1757). In this prospective cohort study, a significant improvement in SNOT-22 scores was demonstrated at 3, 12 and 36 months with CRSwNP patients were found to benefit more from surgery than those with CRSsNP. Revision surgery was indicated in 3.6% of patients at 12 months and 11.8% at 36 months (1757).
6.8.3. Efficacy of surgery for nasal polyps compared to CRSsNP
The efficacy of FESS in patients with CRSwNP is at least as great as in patients with CRSsNP
There is some evidence that a significantly higher rate of recurrent surgery is required in patients with nasal polyposis than those without polyps (1887). Despite the increased rates of revision, patients with polyps may have more improvement following sinus surgery than CRSsNP patients (1757). In one large series, FESS was performed in 251 patients with medically refractory CRS (86 with polyps and 165 without), and the patients followed for at least 12 months. Symptom scores improved significantly in both groups (p<0.001). There were no significant differences between the groups except in oropharyngeal symptoms, which were improved more in the non-polyp patients (1888)..
In another series, 43 patients with polyps were compared with 76 patients without polyps before and after ESS. Mean follow-up was 1.5 years. Patients were analysed prospectively based on CT scans, endoscopy, quality-of-life (QOL) assessment and visual analog symptom scales. Despite significantly worse objective scores, patients with polyps surprisingly reported significantly better QOL scores and less facial pain or headache both pre- and postoperatively (1885).
6.8.4. Efficacy of surgery for nasal polyps compared to
medical therapy
The efficacy of FESS is equivalent to the efficacy of medical therapy (which includes systemic corticosterioid treatment) in CRSwNP patients randomized to receive one or other treatment.
As surgery for nasal polyposis is usually not considered until medical therapy has failed to provide adequate symptom relief, a clinically relevant comparison of the relative efficacies of medical and surgical treatment is difficult to make because the patient populations in whom these treatment modalities are indicated are different.
However, if untreated patients are randomized into either a medical treatment or surgical arm comparisons of the relative efficacies of these approaches can be made. In a randomized controlled trial comparing the effect of medical and surgical treatment of CRS on quality of life, 90 patients were evaluated before and after 6 and 12 months of follow up after either medical or surgical therapy (15). Both medical and surgical treatment of CRS significantly improved almost all the domains of SNOT 20 and SF-36 (p < 0.05), with no significant difference being found between the medical and surgical groups (p > 0.05). The presence of nasal polyps did not adversely affect the outcome observed after either medical or surgical treatment. Another study included 109 patients with nasal polyps (1889). A total of 53 patients were randomly allocated to receive oral prednisone for 2 weeks and 56 patients were allocated to undergo ESS. All patients were administered intranasal budesonide for 12 months. Patients were evaluated for nasal symptoms, polyp size and quality of life. At 6 and 12 months, a significant improvement in all SF-36 domains was observed after both medical and surgical treatment, reaching the levels seen in the general population. Nasal symptoms and polyp size improved after both medical and surgical treatment at 6 and 12 months. These results suggest that both medical and surgical treatment can lead to similar effects in improving quality of life.
Although these studies provide an interesting insight into the relative efficacies of medical and surgical therapy in unselected patients, neither reflects currently accepted practice guidelines in which surgery is performed in medically refractory patients.
The efficacy of FESS is equivalent to the efficacy of medical therapy (which includes systemic corticosterioid treatment) in CRSwNP patients randomized to receive one or other treatment.
As surgery for nasal polyposis is usually not considered until medical therapy has failed to provide adequate symptom relief, a clinically relevant comparison of the relative efficacies of medical and surgical treatment is difficult to make because the patient populations in whom these treatment modalities are indicated are different.
However, if untreated patients are randomized into either a medical treatment or surgical arm comparisons of the relative efficacies of these approaches can be made. In a randomized controlled trial comparing the effect of medical and surgical treatment of CRS on quality of life, 90 patients were evaluated before and after 6 and 12 months of follow up after either medical or surgical therapy (15). Both medical and surgical treatment of CRS significantly improved almost all the domains of SNOT 20 and SF-36 (p < 0.05), with no significant difference being found between the medical and surgical groups (p > 0.05). The presence of nasal polyps did not adversely affect the outcome observed after either medical or surgical treatment. Another study included 109 patients with nasal polyps (1889). A total of 53 patients were randomly allocated to receive oral prednisone for 2 weeks and 56 patients were allocated to undergo ESS. All patients were administered intranasal budesonide for 12 months. Patients were evaluated for nasal symptoms, polyp size and quality of life. At 6 and 12 months, a significant improvement in all SF-36 domains was observed after both medical and surgical treatment, reaching the levels seen in the general population. Nasal symptoms and polyp size improved after both medical and surgical treatment at 6 and 12 months. These results suggest that both medical and surgical treatment can lead to similar effects in improving quality of life.
Although these studies provide an interesting insight into the relative efficacies of medical and surgical therapy in unselected patients, neither reflects currently accepted practice guidelines in which surgery is performed in medically refractory patients.
6.8.5. Extent of treatment
The extent of surgery required to optimize outcomes in CRSwNP patients has not been established. Some reports suggest that outcomes may be improved after more extensive procedures.
A wide range of surgical procedures are undertaken to treat CRS and currently the vast majority of these are being performed endonasally. Although treatment by polypectomy alone effectively relieves symptoms of nasal blockage, it is associated with high recurrence rates (1887, 1890). In 1997, Jankowski et al. prospectively compared patient satisfaction and recurrence rate of nasal polyps in a group of patients with severe nasal polyposis, 39 of whom had radical ethmoidectomy (nasalisation) and 37 of whom underwent functional ethmoidectomy performed by two different surgeons (reducing comparability between the groups) (1767). It was found that the nasalisation group had a significantly lower recurrence rate of 22.7% versus 58.3% in the functional ethmoidectomy group. The overall functional benefit was also reported to be significantly higher in the nasalisation group, suggesting that treatment of nasal polyposis with complete ethmoidectomy leads to better longterm results than incomplete ethmoidectomy.
In a more recent study a retrospective review of revision rates and complications in 149 patients who underwent extensive FESS was performed (1891). A comparison was made with patients from the UK National Comparative Audit who underwent polyp surgery limited to the anterior ethmoid cavity. At 36 months after surgery, five patients from the extensive surgery group had undergone a revision procedure, which was significantly less than the National Audit figure (4.0 vs. 12.3%, P = 0.006). The peri-operative adverse complication rate was similar (7.4 vs. 6.6%). There was a large improvement in SNOT-22 scores from the pre-operative period (mean 39) to the post-operative period (mean 8) in the extensive surgery group. This study provides some evidence that extensive sinus surgery performed by an experienced rhinologist can lead to a lower revision rate without compromising patient safety.
6.8.5.1. Surgery of the frontal recess
Mucosal thickening of the frontal recess easily leads to obstruction of the frontal sinus outflow tract. At the time of initial development of endoscopic surgery there was a reluctance to perform frontal recess dissection because of the ease with which the recess may stenose with postoperative scarring. However in recent years understanding of the anatomy of this region and instruments for its dissection have improved, and impressive patency rates have been reported. A recent review of the evidence of clinical efficacy of frontal sinus procedures for CRS found a generally high rate of success but some of the series were small and the follow up relatively short term (1892). The review did not differentiate between CRS with and without polyps. Chan et al reported results from a group of 58 patients with eosinophilic CRS (most of whom presented clinically with nasal polyps) after frontal sinusotomy (Draf IIa) with an average follow-up of 61.6 months. A very high patency rate of 85% was achieved (although this was slightly lower than the 90% patency rate in non-eosinophilic CRS patients). This series demonstrates that frontal sinusotomy performed by experienced surgeons can produce excellent long-term patency rates (1893). Friedman reported a slightly lower patency rate of 71.1% after a similarly long period of follow up in a group of 152 patients in whom frontal sinusotomy (Draf IIa) was performed (1894). Many of these patients had nasal polyps, and recurrent polyps or scarring were the two most common causes of obstruction of the frontal sinus in this series.
6.8.5.2. Approaches to the maxillary sinus
In an effort to clear polypoid mucosa as completely as possible from the maxillary sinus, anterior antrotomies have been performed to allow access of powered instruments. The efficacy of this manoeuvre on the outcome after FESS has been the subject of a small number of studies. One such trial has compared the results of performing a canine fossa puncture with clearance of polyps via a middle meatal antrostomy (1895). No benefit of the canine fossa procedure over conventional middle meatal antrostomy was seen after 12 months follow up, however the number in both groups is small. The authors concluded that although canine fossa puncture is a useful method for removing severe mucosal disease that cannot be reached through the MMA, it does not guarantee a better subjective or objective surgical outcome in patients with nasal polyposis. However, case control studies have found that patients who had a canine fossa puncture had a better outcome than those with similar disease severity who did not (1896, 1897). Another approach to chronically diseased maxillary antra has been to lower the medial antral wall to the level of the hard palate. This procedure necessitates at least partial removal of the inferior turbinate and has been termed a mega-antrotomy. Cho and Hwang reported on a series of 28 patients who underwent 42 mega-antrostomies for recalcitrant maxillary sinusitis (1457). All patients had previous maxillary sinus surgery (mean number of procedures 2.3). At the time of the most recent postoperative examination, 74% of patients reported complete resolution of symptoms while 26% reported partial symptomatic improvement. There were no complications and the revision rate was 0%.
The extent of surgery required to optimize outcomes in CRSwNP patients has not been established. Some reports suggest that outcomes may be improved after more extensive procedures.
A wide range of surgical procedures are undertaken to treat CRS and currently the vast majority of these are being performed endonasally. Although treatment by polypectomy alone effectively relieves symptoms of nasal blockage, it is associated with high recurrence rates (1887, 1890). In 1997, Jankowski et al. prospectively compared patient satisfaction and recurrence rate of nasal polyps in a group of patients with severe nasal polyposis, 39 of whom had radical ethmoidectomy (nasalisation) and 37 of whom underwent functional ethmoidectomy performed by two different surgeons (reducing comparability between the groups) (1767). It was found that the nasalisation group had a significantly lower recurrence rate of 22.7% versus 58.3% in the functional ethmoidectomy group. The overall functional benefit was also reported to be significantly higher in the nasalisation group, suggesting that treatment of nasal polyposis with complete ethmoidectomy leads to better longterm results than incomplete ethmoidectomy.
In a more recent study a retrospective review of revision rates and complications in 149 patients who underwent extensive FESS was performed (1891). A comparison was made with patients from the UK National Comparative Audit who underwent polyp surgery limited to the anterior ethmoid cavity. At 36 months after surgery, five patients from the extensive surgery group had undergone a revision procedure, which was significantly less than the National Audit figure (4.0 vs. 12.3%, P = 0.006). The peri-operative adverse complication rate was similar (7.4 vs. 6.6%). There was a large improvement in SNOT-22 scores from the pre-operative period (mean 39) to the post-operative period (mean 8) in the extensive surgery group. This study provides some evidence that extensive sinus surgery performed by an experienced rhinologist can lead to a lower revision rate without compromising patient safety.
6.8.5.1. Surgery of the frontal recess
Mucosal thickening of the frontal recess easily leads to obstruction of the frontal sinus outflow tract. At the time of initial development of endoscopic surgery there was a reluctance to perform frontal recess dissection because of the ease with which the recess may stenose with postoperative scarring. However in recent years understanding of the anatomy of this region and instruments for its dissection have improved, and impressive patency rates have been reported. A recent review of the evidence of clinical efficacy of frontal sinus procedures for CRS found a generally high rate of success but some of the series were small and the follow up relatively short term (1892). The review did not differentiate between CRS with and without polyps. Chan et al reported results from a group of 58 patients with eosinophilic CRS (most of whom presented clinically with nasal polyps) after frontal sinusotomy (Draf IIa) with an average follow-up of 61.6 months. A very high patency rate of 85% was achieved (although this was slightly lower than the 90% patency rate in non-eosinophilic CRS patients). This series demonstrates that frontal sinusotomy performed by experienced surgeons can produce excellent long-term patency rates (1893). Friedman reported a slightly lower patency rate of 71.1% after a similarly long period of follow up in a group of 152 patients in whom frontal sinusotomy (Draf IIa) was performed (1894). Many of these patients had nasal polyps, and recurrent polyps or scarring were the two most common causes of obstruction of the frontal sinus in this series.
6.8.5.2. Approaches to the maxillary sinus
In an effort to clear polypoid mucosa as completely as possible from the maxillary sinus, anterior antrotomies have been performed to allow access of powered instruments. The efficacy of this manoeuvre on the outcome after FESS has been the subject of a small number of studies. One such trial has compared the results of performing a canine fossa puncture with clearance of polyps via a middle meatal antrostomy (1895). No benefit of the canine fossa procedure over conventional middle meatal antrostomy was seen after 12 months follow up, however the number in both groups is small. The authors concluded that although canine fossa puncture is a useful method for removing severe mucosal disease that cannot be reached through the MMA, it does not guarantee a better subjective or objective surgical outcome in patients with nasal polyposis. However, case control studies have found that patients who had a canine fossa puncture had a better outcome than those with similar disease severity who did not (1896, 1897). Another approach to chronically diseased maxillary antra has been to lower the medial antral wall to the level of the hard palate. This procedure necessitates at least partial removal of the inferior turbinate and has been termed a mega-antrotomy. Cho and Hwang reported on a series of 28 patients who underwent 42 mega-antrostomies for recalcitrant maxillary sinusitis (1457). All patients had previous maxillary sinus surgery (mean number of procedures 2.3). At the time of the most recent postoperative examination, 74% of patients reported complete resolution of symptoms while 26% reported partial symptomatic improvement. There were no complications and the revision rate was 0%.
6.8.6. Perioperative medical care
Prolonged postoperative medical treatment with topical corticosteroid sprays would appear to improve outcomes post FESS for CRSwNP
Although many studies have demonstrated the effectiveness of sinus surgery for patients who have nasal polyposis, it should not be thought of as the only treatment but rather as a modality used to manage patients to remove the disease burden and increase the efficacy of post-operative medical therapy. Surgically removed polyps have a high tendency for recurrence without aggressive postoperative medical management.
However, surgical management can be used to decrease the amount of inflammation so that the medical treatment may become more effective and the rate of recurrence may be reduced. In one study, 109 patients (77 of whom had nasal polyps) were randomized to receive postoperative fluticasone spray beginning six weeks after FESS. The change in the overall visual analogue score was significantly better in the fluticasone group at 5 years and significantly more prednisolone rescue medication courses were prescribed in the placebo group (1821).
There is evidence that administration of systemic steroids in the postoperative period for patients who have polyps may have a significant impact on their postoperative course. In a randomized placebo controlled study, those patients who received a course of perioperative prednisone (for five preoperative and 9 post operative days) had significantly healthier looking cavities at 6 month follow up than those patients who received the placebo (1898). There was however no impact of perioperative prednisone on symptom scores.
6.8.7. Efficacy of revision surgery for nasal polyps
Revision surgery may be performed with good outcomes for recurrent nasal polyposis. Recalcitrant frontal sinus disease can be treated with good success rates and relatively little morbidity by performing the endoscopic modified Lothrop procedure
Even after meticulous removal of polyps and polypoid mucosa, the opening of all sinus ostia to their anatomical limits and optimal postoperative medical care, some patients will present with recurrent disease. The frontal recess is the most common site of recurrence, probably because it is so easily stenosed postoperatively with scarring and recurrent inflammation.
Many studies have examined the prognostic factors affecting the success of endoscopic sinus surgery (ESS), and a history of previous ESS is often found to be a factor contributing to a poor surgical outcome. However this is not uniformly the case. In a recently reported study, the postoperative results between primary (101 cases) and revision (24 cases) FESS for chronic rhinosinusitis with nasal polyposis were compared using the SNOT-20 and nasal endoscopy scores at 6 and 12 months (1895). Postoperatively the subjective and objective surgical outcomes of the 2 groups did not differ statistically. Also the need for additional medications during the follow-up period and the proportion of patients who required additional surgical intervention due to surgical failure was similar in both groups.
The extent of revision surgery is largely guided by postoperative CT scan results. If persisting sinus cells or septations are causing ongoing obstruction or stenosis of sinus ostia, then these require removal. However, in cases of severe nasal polyposis it is not uncommon for the frontal recess to have been completely cleared of cells, but for the soft tissue or neo-osteogenesis to have narrowed or occluded the recess. The implication is that the frontal recess needs to be enlarged beyond its anatomical limits. During the endoscopic modified Lothrop procedure the floor of the frontal sinuses and the intersinus septum are removed, creating a large common ostium (1899). A very recently published series of 122 consecutive patients undergoing an endoscopic modified Lothrop procedure, reported a frontal patency rate of 90%(1900). A meta-analysis of the 612 cases of endoscopic modified Lothrop procedures has been reported recently (1901). Nearly 30% of these patients had nasal polyposis. In those patients with available data, patency was achieved in 95.9% and improvement of symptoms in 82.2%. The overall failure rate (requirement of further surgery) was 13.9%. The reported complication was low. This meta-analysis suggests that the endoscopic modified Lothrop procedure is a very good option if the frontal sinusitis persists after frontal sinusotomy has been performed. It would appear to offer a success rate similar to frontal sinus obliteration procedures but with much less morbidity.
6.8.8. Complications of surgical treatment of nasal polyps
The frequency of occurrence of severe orbital or skull base complications is very low in recently reported series
A number of significant complications have been reported after FESS for nasal polyps. Fortunately the frequency of occurrence of severe complications would appear to be reducing with time, and the risk of major orbital, intracranial or vascular injury occurring is now very low.
A systematic review of safety and efficacy of FESS for removal of polyps by Dalziel et al. in 2006 reviewed three randomized control trials, four nonrandomized comparative studies and 35 case series studies (1902). Major complications ranged from 0% to 1.5% and minor complications from 1.1% to 20.8%. Infection was reported in 16% of FESS procedures and 28% of conventional procedures. Disease recurrence ranged from 4% to 60% with a median of 20% across all studies reviewed. Recurrence following revision surgery ranged from 3% to 42% with a median of 6%.
The National Audit in England and Wales assessed the rate of complications of surgery for polyposis and chronic rhinosinusitis (1903). A total of 3123 patients were included in the study of which 2176 (69.1%) had nasal polyps. Nearly 40% of patients underwent a simple polypectomy ± antral washout and the majority of operations were performed endoscopically. The microdebrider was used in 16.5% of operations. Major complications were observed in 0.4% of cases, minor complications in 6.6%. Statistical significance was found in complication rates between grades of polyposis, use of microdebrider, increasing Lund-Mackay score, increasing American Society of Anesthesiology score and patients with previous sinonasal surgery.
A retrospective study by Ecevit et al. compared the rate of microdebrider complications between 90 cases (177 sides) of chronic sinusitis with polyps to 49 cases (98 sides) of chronic sinusitis without polyps (1904). The minor and major complication rate for the group with nasal polyps was 11.8 and 0.5% respectively. Only one major complication was reported, a cerebrospinal fistula which was repaired intra-operatively. The complication rate for chronic sinusitis without polyps was 4%. The difference between complication rates of the two groups was statistically significant (p=0.0001) A retrospective medical record review by Devars du Mayne et al. assessed outcomes of patients with nasal polyps undergoing either radical ethmoidectomy (n=77) or polypectomy (n=50) (1905). No severe complications were observed in either group although few complications were seen in the polypectomy group (8% vs 18.3%). Seven patients from the radical ethmoidectomy group required further surgery (four for polyp recurrence, two for ethmoidofrontal mucocoele and one for nasofrontal duct stenosis) and four from the polypectomy group required further surgery, all for polyp recurrence.
A retrospective case note review was performed by Bajaj et al. assessing the results of FESS as day-case surgery (1906). Of the 105 procedures, 62.8% had both CRS and NP. The only reported complication of the study was bleeding, seen in 7 patients. Five patients had primary haemorrhage and were packed in theatre and 2 had reactionary bleeding, 1 of which required packing.
Prolonged postoperative medical treatment with topical corticosteroid sprays would appear to improve outcomes post FESS for CRSwNP
Although many studies have demonstrated the effectiveness of sinus surgery for patients who have nasal polyposis, it should not be thought of as the only treatment but rather as a modality used to manage patients to remove the disease burden and increase the efficacy of post-operative medical therapy. Surgically removed polyps have a high tendency for recurrence without aggressive postoperative medical management.
However, surgical management can be used to decrease the amount of inflammation so that the medical treatment may become more effective and the rate of recurrence may be reduced. In one study, 109 patients (77 of whom had nasal polyps) were randomized to receive postoperative fluticasone spray beginning six weeks after FESS. The change in the overall visual analogue score was significantly better in the fluticasone group at 5 years and significantly more prednisolone rescue medication courses were prescribed in the placebo group (1821).
There is evidence that administration of systemic steroids in the postoperative period for patients who have polyps may have a significant impact on their postoperative course. In a randomized placebo controlled study, those patients who received a course of perioperative prednisone (for five preoperative and 9 post operative days) had significantly healthier looking cavities at 6 month follow up than those patients who received the placebo (1898). There was however no impact of perioperative prednisone on symptom scores.
6.8.7. Efficacy of revision surgery for nasal polyps
Revision surgery may be performed with good outcomes for recurrent nasal polyposis. Recalcitrant frontal sinus disease can be treated with good success rates and relatively little morbidity by performing the endoscopic modified Lothrop procedure
Even after meticulous removal of polyps and polypoid mucosa, the opening of all sinus ostia to their anatomical limits and optimal postoperative medical care, some patients will present with recurrent disease. The frontal recess is the most common site of recurrence, probably because it is so easily stenosed postoperatively with scarring and recurrent inflammation.
Many studies have examined the prognostic factors affecting the success of endoscopic sinus surgery (ESS), and a history of previous ESS is often found to be a factor contributing to a poor surgical outcome. However this is not uniformly the case. In a recently reported study, the postoperative results between primary (101 cases) and revision (24 cases) FESS for chronic rhinosinusitis with nasal polyposis were compared using the SNOT-20 and nasal endoscopy scores at 6 and 12 months (1895). Postoperatively the subjective and objective surgical outcomes of the 2 groups did not differ statistically. Also the need for additional medications during the follow-up period and the proportion of patients who required additional surgical intervention due to surgical failure was similar in both groups.
The extent of revision surgery is largely guided by postoperative CT scan results. If persisting sinus cells or septations are causing ongoing obstruction or stenosis of sinus ostia, then these require removal. However, in cases of severe nasal polyposis it is not uncommon for the frontal recess to have been completely cleared of cells, but for the soft tissue or neo-osteogenesis to have narrowed or occluded the recess. The implication is that the frontal recess needs to be enlarged beyond its anatomical limits. During the endoscopic modified Lothrop procedure the floor of the frontal sinuses and the intersinus septum are removed, creating a large common ostium (1899). A very recently published series of 122 consecutive patients undergoing an endoscopic modified Lothrop procedure, reported a frontal patency rate of 90%(1900). A meta-analysis of the 612 cases of endoscopic modified Lothrop procedures has been reported recently (1901). Nearly 30% of these patients had nasal polyposis. In those patients with available data, patency was achieved in 95.9% and improvement of symptoms in 82.2%. The overall failure rate (requirement of further surgery) was 13.9%. The reported complication was low. This meta-analysis suggests that the endoscopic modified Lothrop procedure is a very good option if the frontal sinusitis persists after frontal sinusotomy has been performed. It would appear to offer a success rate similar to frontal sinus obliteration procedures but with much less morbidity.
6.8.8. Complications of surgical treatment of nasal polyps
The frequency of occurrence of severe orbital or skull base complications is very low in recently reported series
A number of significant complications have been reported after FESS for nasal polyps. Fortunately the frequency of occurrence of severe complications would appear to be reducing with time, and the risk of major orbital, intracranial or vascular injury occurring is now very low.
A systematic review of safety and efficacy of FESS for removal of polyps by Dalziel et al. in 2006 reviewed three randomized control trials, four nonrandomized comparative studies and 35 case series studies (1902). Major complications ranged from 0% to 1.5% and minor complications from 1.1% to 20.8%. Infection was reported in 16% of FESS procedures and 28% of conventional procedures. Disease recurrence ranged from 4% to 60% with a median of 20% across all studies reviewed. Recurrence following revision surgery ranged from 3% to 42% with a median of 6%.
The National Audit in England and Wales assessed the rate of complications of surgery for polyposis and chronic rhinosinusitis (1903). A total of 3123 patients were included in the study of which 2176 (69.1%) had nasal polyps. Nearly 40% of patients underwent a simple polypectomy ± antral washout and the majority of operations were performed endoscopically. The microdebrider was used in 16.5% of operations. Major complications were observed in 0.4% of cases, minor complications in 6.6%. Statistical significance was found in complication rates between grades of polyposis, use of microdebrider, increasing Lund-Mackay score, increasing American Society of Anesthesiology score and patients with previous sinonasal surgery.
A retrospective study by Ecevit et al. compared the rate of microdebrider complications between 90 cases (177 sides) of chronic sinusitis with polyps to 49 cases (98 sides) of chronic sinusitis without polyps (1904). The minor and major complication rate for the group with nasal polyps was 11.8 and 0.5% respectively. Only one major complication was reported, a cerebrospinal fistula which was repaired intra-operatively. The complication rate for chronic sinusitis without polyps was 4%. The difference between complication rates of the two groups was statistically significant (p=0.0001) A retrospective medical record review by Devars du Mayne et al. assessed outcomes of patients with nasal polyps undergoing either radical ethmoidectomy (n=77) or polypectomy (n=50) (1905). No severe complications were observed in either group although few complications were seen in the polypectomy group (8% vs 18.3%). Seven patients from the radical ethmoidectomy group required further surgery (four for polyp recurrence, two for ethmoidofrontal mucocoele and one for nasofrontal duct stenosis) and four from the polypectomy group required further surgery, all for polyp recurrence.
A retrospective case note review was performed by Bajaj et al. assessing the results of FESS as day-case surgery (1906). Of the 105 procedures, 62.8% had both CRS and NP. The only reported complication of the study was bleeding, seen in 7 patients. Five patients had primary haemorrhage and were packed in theatre and 2 had reactionary bleeding, 1 of which required packing.
6.9. Influence of concomitant diseases on outcome of
treatment in Chronic Rhinosinusitis with and without NP
including reasons for failure of medical and surgical
therapy
6.9.1. Summary
Many factors potentially outcome of treatment in CRS with and without nasal polyps. Extent of the disease, asthma, AERD, CF and biofilm formation have been proven to have a negative influence. For some factors, like allergy, smoking and , type of inflammation, studies contradict each other. Gender does not seem to influence the results of treatment of CRS. Patients with higher age and fatigue may have a more pronounced improvement after FESS
6.9.2. Sinus surgery in the elderly
In general no difference is found in symptomatology and QoL of CRS in the elderly (1187, 1907, 1908). In a study comparing the objective endoscopic findings and subjective improvements in symptoms among the groups 6 months after the functional endoscopic sinus surgery (FESS) in three groups according to patient age: 20 paediatric (5-18 yr), 20 adult (19-65 yr), and 20 geriatric patients (over 65 yr.) no statistical differences in polyp extent or Lund-Mackay score were found before FESS between the three age groups and the subjective surgical outcome did not differ statistically between the groups, with the exception of olfactory disturbance. On the other hand the objective surgical outcome based on the endoscopic findings was worst in the paediatric group (45%), whereas the geriatric group showed the best results (90%). The differences in objective outcome among the three groups were significant, and patient age was a predictive variable for surgical result based on multiple logistic regression analysis (1907). Also in the study of Sil et al. increasing age was significantly positively correlated with the objective signs improvement in endoscopic polyp scores and in nasal mucociliary clearance times, but not in symptomatology (1909). This better objective outcome in the elderly could not be substantiated by Reh, however his elderly group comprised of only 18 patients (1908). Analysis of recurrences was accomplished in a retrospective study on 192 patients operated for CRSwNP. No association of recurrence with age, gender, purulent nasal discharge, facial pain, anosmia, post nasal dripping, headache, nasal allergy, and asthma were observed (1910).
In a retrospective case control study, FESS outcome in 46 CRS patients > 65 years were compared with 522 CRS patients who were 18¬64 years old (1911). In the elder patient group, complications occurred significantly more frequently than in the younger patients group. In particular orbital complications were frequently observed in the elder patient group (level III). Jiang and Su retrospectively compared complication rates of 171 CRS patients older than 65 years with 837 adult patients and 104 patients younger than 16 years. They found that the geriatric group experienced a disproportionately larger share of operative complications. Outcomes were similar in all three groups (1912). A study evaluated outcome of sinus surgery in 180 patients older than 65 yrs. compared to 180 adults (15-65 yrs.), both groups with CRS. Diabetes mellitus was shown to be risk factor for complications, not so much the patients' age (1913).
Conclusion: CRS is a common condition in the elderly. Reported symptomatology before and after surgery does not differ from a younger patient population and postoperative objective signs seem to improve more in the elderly. However, higher surgical complication rates were found in 2 reports. Moreover, general anaesthesia bears higher risks and the capacity to recover from a severe surgical complication such as a CSF leak may be impaired.
6.9.3. Gender
In most studies women with CRS report higher levels of symptoms despite less extensive disease and this is likely to be due to a systematic difference in response style (1187, 1914, 1915). In a prospective study of 514 adult patients who presented with chronic rhinosinusitis with and without nasal polyposis facial pain and headache were more prevalent among women, while nasal obstruction was more prevalent among men. This is partly explained by the fact that CRSsNP was the more common diagnosis among women, while CRSwNP was the more common diagnosis among men. There was no statistically significant difference in the improvement of the other presenting symptoms, comparing the gender (1915). Most other studies also show comparable improvement of FESS between men and women (1909, 1910, 1915).
6.9.4. Extent of disease at baseline
Patients presenting with extensive disease suggested by C.T scan staging are at higher risk for the development of recurrences after endonasal surgery for nasal polyps (1910). CT scan scores and polyp scores were the strongest predictors of the need for postoperative systemic medication (1909).
6.9.5. Primary versus revision surgery
The symptomatic relief that revision FESS can provide for patients with refractory chronic rhinosinusitis is similar to that following a primary FESS (1785, 1918). However, in one study patients undergoing primary surgery were 2 times more likely to improve compared with patients undergoing revision surgery (1189).
6.9.6. Type of inflammation
The influence of the type of inflammation on treatment is contradictory
The efficacy of macrolides appears to be less in patients with CRSwNP, severe findings on CT scans, asthma, low IgE and polyps with increased eosinophil infiltration (1708, 1713). There is a significant positive correlation between sinus CT stage and peripheral eosinophil levels. Eighty-nine percent of the abnormal eosinophil counts (>550 cells/microL) were associated with CT scores higher than 12. Total IgE did not correlate with CT stage of disease (1919).
Patients with a total peripheral eosinophil count of 520/microl or more or mucosal eosinophilia were more likely to experience recurrence of CRS after surgery in two studies (878, 1920). However in another study CRS patients with higher levels of eosinophils were less likely to suffer from post-operative recurrent sinonasal disease when treated post-operatively with nasal corticosteroids (27).
6.9.1. Summary
Many factors potentially outcome of treatment in CRS with and without nasal polyps. Extent of the disease, asthma, AERD, CF and biofilm formation have been proven to have a negative influence. For some factors, like allergy, smoking and , type of inflammation, studies contradict each other. Gender does not seem to influence the results of treatment of CRS. Patients with higher age and fatigue may have a more pronounced improvement after FESS
6.9.2. Sinus surgery in the elderly
In general no difference is found in symptomatology and QoL of CRS in the elderly (1187, 1907, 1908). In a study comparing the objective endoscopic findings and subjective improvements in symptoms among the groups 6 months after the functional endoscopic sinus surgery (FESS) in three groups according to patient age: 20 paediatric (5-18 yr), 20 adult (19-65 yr), and 20 geriatric patients (over 65 yr.) no statistical differences in polyp extent or Lund-Mackay score were found before FESS between the three age groups and the subjective surgical outcome did not differ statistically between the groups, with the exception of olfactory disturbance. On the other hand the objective surgical outcome based on the endoscopic findings was worst in the paediatric group (45%), whereas the geriatric group showed the best results (90%). The differences in objective outcome among the three groups were significant, and patient age was a predictive variable for surgical result based on multiple logistic regression analysis (1907). Also in the study of Sil et al. increasing age was significantly positively correlated with the objective signs improvement in endoscopic polyp scores and in nasal mucociliary clearance times, but not in symptomatology (1909). This better objective outcome in the elderly could not be substantiated by Reh, however his elderly group comprised of only 18 patients (1908). Analysis of recurrences was accomplished in a retrospective study on 192 patients operated for CRSwNP. No association of recurrence with age, gender, purulent nasal discharge, facial pain, anosmia, post nasal dripping, headache, nasal allergy, and asthma were observed (1910).
In a retrospective case control study, FESS outcome in 46 CRS patients > 65 years were compared with 522 CRS patients who were 18¬64 years old (1911). In the elder patient group, complications occurred significantly more frequently than in the younger patients group. In particular orbital complications were frequently observed in the elder patient group (level III). Jiang and Su retrospectively compared complication rates of 171 CRS patients older than 65 years with 837 adult patients and 104 patients younger than 16 years. They found that the geriatric group experienced a disproportionately larger share of operative complications. Outcomes were similar in all three groups (1912). A study evaluated outcome of sinus surgery in 180 patients older than 65 yrs. compared to 180 adults (15-65 yrs.), both groups with CRS. Diabetes mellitus was shown to be risk factor for complications, not so much the patients' age (1913).
Conclusion: CRS is a common condition in the elderly. Reported symptomatology before and after surgery does not differ from a younger patient population and postoperative objective signs seem to improve more in the elderly. However, higher surgical complication rates were found in 2 reports. Moreover, general anaesthesia bears higher risks and the capacity to recover from a severe surgical complication such as a CSF leak may be impaired.
6.9.3. Gender
In most studies women with CRS report higher levels of symptoms despite less extensive disease and this is likely to be due to a systematic difference in response style (1187, 1914, 1915). In a prospective study of 514 adult patients who presented with chronic rhinosinusitis with and without nasal polyposis facial pain and headache were more prevalent among women, while nasal obstruction was more prevalent among men. This is partly explained by the fact that CRSsNP was the more common diagnosis among women, while CRSwNP was the more common diagnosis among men. There was no statistically significant difference in the improvement of the other presenting symptoms, comparing the gender (1915). Most other studies also show comparable improvement of FESS between men and women (1909, 1910, 1915).
6.9.4. Extent of disease at baseline
Patients presenting with extensive disease suggested by C.T scan staging are at higher risk for the development of recurrences after endonasal surgery for nasal polyps (1910). CT scan scores and polyp scores were the strongest predictors of the need for postoperative systemic medication (1909).
6.9.5. Primary versus revision surgery
The symptomatic relief that revision FESS can provide for patients with refractory chronic rhinosinusitis is similar to that following a primary FESS (1785, 1918). However, in one study patients undergoing primary surgery were 2 times more likely to improve compared with patients undergoing revision surgery (1189).
6.9.6. Type of inflammation
The influence of the type of inflammation on treatment is contradictory
The efficacy of macrolides appears to be less in patients with CRSwNP, severe findings on CT scans, asthma, low IgE and polyps with increased eosinophil infiltration (1708, 1713). There is a significant positive correlation between sinus CT stage and peripheral eosinophil levels. Eighty-nine percent of the abnormal eosinophil counts (>550 cells/microL) were associated with CT scores higher than 12. Total IgE did not correlate with CT stage of disease (1919).
Patients with a total peripheral eosinophil count of 520/microl or more or mucosal eosinophilia were more likely to experience recurrence of CRS after surgery in two studies (878, 1920). However in another study CRS patients with higher levels of eosinophils were less likely to suffer from post-operative recurrent sinonasal disease when treated post-operatively with nasal corticosteroids (27).
6.9.7. Asthma
Bronchial asthma is frequently associated with CRS with and without polyps and may have influence on sinus surgery outcomes. Prevalence of asthma is shown to be much higher in patients with CRS than in normal population. A study of 145 consecutive adult CRS patients evaluated the prevalence of asthma with CRS. The study showed 23,4% prevalence of asthma compared to the 5% in adult general population. These patients had also significantly higher prevalence of polyps (22%, p=0.004), olfactory dysfunction (6%, p=0.001) and nasal congestion (60%, p=0.037). There was no difference between CRS patients with or without asthma in the proportion of patients needing primary sinus surgery , but patients with asthma did require significantly more revision sinus surgeries (mean 2.9 vs 1.5) p=0.0003 (1921).
More severe sinus disease in CRS patients with concomitant asthma has been reported (746, 762, 1922, 1923). Clinically, CRS patients with polyps and asthma have higher CT-scores, more severe nasal obstruction and hyposmia, and more severe asthma, while CRS patients without polyps and asthma experience more severe headache and postnasal discharge (746, 1922-1924).The incidence of self reported rhinosinusitis in asthma patients was recently evaluated employing the data of two major asthma trials (1925). Self reported rhinosinusitis was associated with bronchial asthma in 70% of the 2500 study participants. Asthma patients with concomitant rhinosinusitis had more asthma exacerbations, worse asthma symptoms, worse cough, and worse sleep quality. Investigations of concomitant asthma on sinus surgery outcomes in CRS patients with or without nasal polyps yielded inconsistent results (1417). Concomitant asthma was associated with worse postoperative endoscopy findings in two retrospective analyses (1761, 1775), but had no independent influence on other outcome parameters (level IV). Consistently, symptom scores improved significantly in both asthmatics and non-asthmatics postoperatively, but asthmatics exhibited significantly worse postoperative endoscopic. Asthma with and without aspirin intolerance was shown to be a determinant of recurrence after FESS in patients with CRSsNP and CRSwNP (878, 1521, 1923), but not in all studies (1910, 1926).
6.9.7.1. Asthma in CRSsNP
There is a strong association of asthma with CRS (adjusted OR: 3.47; 95% CI: 3.20-3.76) at all ages (1927). Concomitant asthma is frequent in CRSsNP patients (122). Asthma was shown to be a determinant of recurrence after FESS in patients with CRSsNP (878, 1923), but not in all studies (1926).
6.9.7.2. Asthma in CRSwNP
Asthma is more prevalent in white patients with CRSwNP than in patients with CRSsNP, however the same does not seem to hold for chinese polyps (585, 621). Asthma with and without aspirin intolerance was shown to be a determinant of recurrence after FESS especially in patients with CRSwNP (878, 1521, 1923), but not in all studies (1910, 1926).
6.9.7.3. Effect of treatment on bronchial asthma
The question, how sinus surgery and medical CRS treatment may alter the course of bronchial asthma, was reviewed by Lund (1928) and Scadding (1929). The authors describe the somewhat intricate base of evidence and conclude that the weight of evidence suggests a beneficial effect. Studies published thereafter support this view (1416, 1924, 1930, 1931). In a case series study, 50 CRS patients with concomitant asthma were included (1924). Ragab and co-workers report a prospective evaluation of a subgroup of 43 asthma patients joining a randomised trial comparing the effects of sinus surgery and medical treatment in CRS patients with and without polyps (1423). Outcome parameters included asthma symptoms, control, forced expiratory volume in one second (FEV1), peak flow, exhaled nitric oxide, medication use and hospitalisation at 6 and 12 months from the start of the study. Overall asthma control improved significantly following both treatment modalities, but was better maintained after medical therapy, where improvement could also be demonstrated in the subgroup with nasal polyps. Medical treatment was superior to surgery with respect to a decrease in exhaled nitric oxide and increase in FEV1 in the polyp patients. Two patients noted worsening of asthma postoperatively. Treatment of chronic rhinosinusitis, medical or surgical, benefits concomitant asthma; that associated with nasal polyposis benefits more from medical therapy (level Ib). Haruna and coworkers also showed that asthma was negative factor in the treatment with macrolides (1713).
Palmer and coworkers retrospectively reviewed the charts of a subgroup of 15 CRS patients with steroid dependent asthma selected from a group of 75 consecutive CRS patients with asthma who underwent endoscopic sinus surgery (1932). Outcome parameters included the number of days and total dose of oral prednisone and antibiotics in the year before and after sinus surgery. Fourteen of the 15 patients meeting study criteria decreased their postoperative prednisone requirement by total number of days Antibiotic use also decreased (p < 0.045), with an average use of antibiotic nine weeks preoperatively versus seven weeks postoperatively (Evidence level IV).
Conclusion
Apparently, various confounders not yet sufficiently defined influence the effects of surgical CRS treatment on concomitant asthma. In studies published in recent years, predominantly positive effects of surgical CRS treatment on concomitant asthma severity were reported However, the level of evidence is low (122).
Bronchial asthma is frequently associated with CRS with and without polyps and may have influence on sinus surgery outcomes. Prevalence of asthma is shown to be much higher in patients with CRS than in normal population. A study of 145 consecutive adult CRS patients evaluated the prevalence of asthma with CRS. The study showed 23,4% prevalence of asthma compared to the 5% in adult general population. These patients had also significantly higher prevalence of polyps (22%, p=0.004), olfactory dysfunction (6%, p=0.001) and nasal congestion (60%, p=0.037). There was no difference between CRS patients with or without asthma in the proportion of patients needing primary sinus surgery , but patients with asthma did require significantly more revision sinus surgeries (mean 2.9 vs 1.5) p=0.0003 (1921).
More severe sinus disease in CRS patients with concomitant asthma has been reported (746, 762, 1922, 1923). Clinically, CRS patients with polyps and asthma have higher CT-scores, more severe nasal obstruction and hyposmia, and more severe asthma, while CRS patients without polyps and asthma experience more severe headache and postnasal discharge (746, 1922-1924).The incidence of self reported rhinosinusitis in asthma patients was recently evaluated employing the data of two major asthma trials (1925). Self reported rhinosinusitis was associated with bronchial asthma in 70% of the 2500 study participants. Asthma patients with concomitant rhinosinusitis had more asthma exacerbations, worse asthma symptoms, worse cough, and worse sleep quality. Investigations of concomitant asthma on sinus surgery outcomes in CRS patients with or without nasal polyps yielded inconsistent results (1417). Concomitant asthma was associated with worse postoperative endoscopy findings in two retrospective analyses (1761, 1775), but had no independent influence on other outcome parameters (level IV). Consistently, symptom scores improved significantly in both asthmatics and non-asthmatics postoperatively, but asthmatics exhibited significantly worse postoperative endoscopic. Asthma with and without aspirin intolerance was shown to be a determinant of recurrence after FESS in patients with CRSsNP and CRSwNP (878, 1521, 1923), but not in all studies (1910, 1926).
6.9.7.1. Asthma in CRSsNP
There is a strong association of asthma with CRS (adjusted OR: 3.47; 95% CI: 3.20-3.76) at all ages (1927). Concomitant asthma is frequent in CRSsNP patients (122). Asthma was shown to be a determinant of recurrence after FESS in patients with CRSsNP (878, 1923), but not in all studies (1926).
6.9.7.2. Asthma in CRSwNP
Asthma is more prevalent in white patients with CRSwNP than in patients with CRSsNP, however the same does not seem to hold for chinese polyps (585, 621). Asthma with and without aspirin intolerance was shown to be a determinant of recurrence after FESS especially in patients with CRSwNP (878, 1521, 1923), but not in all studies (1910, 1926).
6.9.7.3. Effect of treatment on bronchial asthma
The question, how sinus surgery and medical CRS treatment may alter the course of bronchial asthma, was reviewed by Lund (1928) and Scadding (1929). The authors describe the somewhat intricate base of evidence and conclude that the weight of evidence suggests a beneficial effect. Studies published thereafter support this view (1416, 1924, 1930, 1931). In a case series study, 50 CRS patients with concomitant asthma were included (1924). Ragab and co-workers report a prospective evaluation of a subgroup of 43 asthma patients joining a randomised trial comparing the effects of sinus surgery and medical treatment in CRS patients with and without polyps (1423). Outcome parameters included asthma symptoms, control, forced expiratory volume in one second (FEV1), peak flow, exhaled nitric oxide, medication use and hospitalisation at 6 and 12 months from the start of the study. Overall asthma control improved significantly following both treatment modalities, but was better maintained after medical therapy, where improvement could also be demonstrated in the subgroup with nasal polyps. Medical treatment was superior to surgery with respect to a decrease in exhaled nitric oxide and increase in FEV1 in the polyp patients. Two patients noted worsening of asthma postoperatively. Treatment of chronic rhinosinusitis, medical or surgical, benefits concomitant asthma; that associated with nasal polyposis benefits more from medical therapy (level Ib). Haruna and coworkers also showed that asthma was negative factor in the treatment with macrolides (1713).
Palmer and coworkers retrospectively reviewed the charts of a subgroup of 15 CRS patients with steroid dependent asthma selected from a group of 75 consecutive CRS patients with asthma who underwent endoscopic sinus surgery (1932). Outcome parameters included the number of days and total dose of oral prednisone and antibiotics in the year before and after sinus surgery. Fourteen of the 15 patients meeting study criteria decreased their postoperative prednisone requirement by total number of days Antibiotic use also decreased (p < 0.045), with an average use of antibiotic nine weeks preoperatively versus seven weeks postoperatively (Evidence level IV).
Conclusion
Apparently, various confounders not yet sufficiently defined influence the effects of surgical CRS treatment on concomitant asthma. In studies published in recent years, predominantly positive effects of surgical CRS treatment on concomitant asthma severity were reported However, the level of evidence is low (122).
6.9.8. Aspirin exacerbated disease (AERD)
The majority of CRS patients with AERD have diffuse, extensive rhinosinusitis (762). AERD patients usually present with more severe asthma (1519). AERD is rather consistently found to adversely affect sinus surgery outcomes (1419, 1420, 1504, 1517, 1933, 1934). The asthmatic complaints of aspirin intolerant and aspirin tolerant patients improved significantly after ESS but CT scan improved more in the aspirin tolerant patients than in the aspirin patients (1519). Although FESS helped both groups of patients, AERD patients had statistically significant better results compared with aspirin tolerant patients in asthma severity scores and decreased need for ICS (1518). The olfactory recovery after FESS for nasal polyposis is significantly affected by the concomitant presence of AERD (1520). Patients with AERD were significantly more likely to have a recurrence and undergo a second surgery following recurrence (risk-odds ratio, 2.7; 95% confidence interval, 1.5 to 3.2; p < 0.01) than were patients without asthma or with only asthma from the triad (1521).
Conclusion
CRS patients with AERD tend to suffer from more extensive sinus disease. They benefit from sinus surgery, but to a lesser extent than patients without AERD. They are more prone to disease recurrence and more frequently undergo revision surgery than aspirin tolerant CRS patients.
6.9.9. Allergy and atopy
In most studies, the diagnosis of allergy was based solely on the presence of a positive skin prick test and/or serum specific IgE determinations. This indicates atopy, but may not suffice to diagnose allergic rhinitis (AR), particularly persistent AR (1933). Consistently, the reported incidence of atopy in CRS patients ranges between 50 and 80%, which is higher than in the general population. The risk-ratio of chronic sinusitis in the AR group in a large cohort was shown to be 4.5 (1935). CRS in atopic patients appears to be more severe (530, 1623, 1936-1940). Atopy was equally frequently associated with CRS with and without polyps (1941). Reports on potential negative effects of allergy on outcomes of surgery are various. There are a number of studies indicating a negative outcome of atopy (1775, 1887, 1900). But also quit some studies did not interference with atopy In recent studies allergy did not seem to be a determinant of treatment failure (1926, 1942- 1944).
6.9.10. Cystic fibrosis
In cystic fibrosis (CF), CRS with and without nasal polyps is observed (1945). The inflammatory profile of CRS in CF patients differs from CRS in patients without CF (18, 1482, 1945). Persistent colonisation with Pseudomonas aeruginosa is a common finding. The paranasal sinuses often harbor distinct bacterial subpopulations, and in the early colonization phases there seems to be a migration from the sinuses to the lower airways, suggesting that independent adaptation and evolution take place in the sinuses. The paranasal sinuses potentially constitute a protected niche of adapted clones of P. aeruginosa, which can intermittently seed the lungs and pave the way for subsequent chronic lung infections (1437).
In 37 patients with cystic fibrosis after lung transplantation, sinus surgery was performed and repeated sinus aspirates and broncho-alveolar lavages were obtained for microbiological examinations. Sinus surgery was successful (three or less Pseudomonas aeruginosa positive aspirates) in 54% and partially successful (4 or 5 positive aspirates) in 27% of patients (1459). A significant correlation of bacterial growth in sinus aspirates and broncho-alveolar lavages was observed (p < 0,0001). Successful sinus management led to a lower incidence of tracheobronchitis and pneumonia (p = 0,009) and a trend toward a lower incidence (p = 0,23) of bronchiolitis obliterans syndrome (Evidence level IV). FESS with subsequent monthly antimicrobial antral lavages (n=32) was compared with a historic control group receiving conventional sinus surgery without postoperative lavages (n=19). The group treated with FESS and antral lavages had fewer operations per patient, and a decrease in repeated surgery at 1 year (10% vs. 47%) and 2 year follow up (22% vs 72%) (Evidence level IV). Not all studies report positive effects of sinus surgery on the lower airways (1946). In general improvement after FESS is significant but quit often recurrences are seen (1947, 1948). While baseline measures of disease severity are worse in the CF population, objective and QoL improvements for adult patients with comorbid CF are comparable to patients without CF (1456).
Conclusion
CF patients frequently suffer from severe CRS, in particular with diffuse polyps refractory to medical treatment. Due to a tendency to recur, repeated sinus surgery is often needed to achieve symptomatic relief. In CF patients, the paranasal sinuses may serve as a source for Pseudomonas aeruginosa induced lung infections. Consequent local antibiotic lavages help to prevent recurrent CRS and lung infection.
The majority of CRS patients with AERD have diffuse, extensive rhinosinusitis (762). AERD patients usually present with more severe asthma (1519). AERD is rather consistently found to adversely affect sinus surgery outcomes (1419, 1420, 1504, 1517, 1933, 1934). The asthmatic complaints of aspirin intolerant and aspirin tolerant patients improved significantly after ESS but CT scan improved more in the aspirin tolerant patients than in the aspirin patients (1519). Although FESS helped both groups of patients, AERD patients had statistically significant better results compared with aspirin tolerant patients in asthma severity scores and decreased need for ICS (1518). The olfactory recovery after FESS for nasal polyposis is significantly affected by the concomitant presence of AERD (1520). Patients with AERD were significantly more likely to have a recurrence and undergo a second surgery following recurrence (risk-odds ratio, 2.7; 95% confidence interval, 1.5 to 3.2; p < 0.01) than were patients without asthma or with only asthma from the triad (1521).
Conclusion
CRS patients with AERD tend to suffer from more extensive sinus disease. They benefit from sinus surgery, but to a lesser extent than patients without AERD. They are more prone to disease recurrence and more frequently undergo revision surgery than aspirin tolerant CRS patients.
6.9.9. Allergy and atopy
In most studies, the diagnosis of allergy was based solely on the presence of a positive skin prick test and/or serum specific IgE determinations. This indicates atopy, but may not suffice to diagnose allergic rhinitis (AR), particularly persistent AR (1933). Consistently, the reported incidence of atopy in CRS patients ranges between 50 and 80%, which is higher than in the general population. The risk-ratio of chronic sinusitis in the AR group in a large cohort was shown to be 4.5 (1935). CRS in atopic patients appears to be more severe (530, 1623, 1936-1940). Atopy was equally frequently associated with CRS with and without polyps (1941). Reports on potential negative effects of allergy on outcomes of surgery are various. There are a number of studies indicating a negative outcome of atopy (1775, 1887, 1900). But also quit some studies did not interference with atopy In recent studies allergy did not seem to be a determinant of treatment failure (1926, 1942- 1944).
6.9.10. Cystic fibrosis
In cystic fibrosis (CF), CRS with and without nasal polyps is observed (1945). The inflammatory profile of CRS in CF patients differs from CRS in patients without CF (18, 1482, 1945). Persistent colonisation with Pseudomonas aeruginosa is a common finding. The paranasal sinuses often harbor distinct bacterial subpopulations, and in the early colonization phases there seems to be a migration from the sinuses to the lower airways, suggesting that independent adaptation and evolution take place in the sinuses. The paranasal sinuses potentially constitute a protected niche of adapted clones of P. aeruginosa, which can intermittently seed the lungs and pave the way for subsequent chronic lung infections (1437).
In 37 patients with cystic fibrosis after lung transplantation, sinus surgery was performed and repeated sinus aspirates and broncho-alveolar lavages were obtained for microbiological examinations. Sinus surgery was successful (three or less Pseudomonas aeruginosa positive aspirates) in 54% and partially successful (4 or 5 positive aspirates) in 27% of patients (1459). A significant correlation of bacterial growth in sinus aspirates and broncho-alveolar lavages was observed (p < 0,0001). Successful sinus management led to a lower incidence of tracheobronchitis and pneumonia (p = 0,009) and a trend toward a lower incidence (p = 0,23) of bronchiolitis obliterans syndrome (Evidence level IV). FESS with subsequent monthly antimicrobial antral lavages (n=32) was compared with a historic control group receiving conventional sinus surgery without postoperative lavages (n=19). The group treated with FESS and antral lavages had fewer operations per patient, and a decrease in repeated surgery at 1 year (10% vs. 47%) and 2 year follow up (22% vs 72%) (Evidence level IV). Not all studies report positive effects of sinus surgery on the lower airways (1946). In general improvement after FESS is significant but quit often recurrences are seen (1947, 1948). While baseline measures of disease severity are worse in the CF population, objective and QoL improvements for adult patients with comorbid CF are comparable to patients without CF (1456).
Conclusion
CF patients frequently suffer from severe CRS, in particular with diffuse polyps refractory to medical treatment. Due to a tendency to recur, repeated sinus surgery is often needed to achieve symptomatic relief. In CF patients, the paranasal sinuses may serve as a source for Pseudomonas aeruginosa induced lung infections. Consequent local antibiotic lavages help to prevent recurrent CRS and lung infection.
6.9.11. Immune dysfunction
Immune deficiency states are frequently associated with CRS include HIV-infection, bone marrow transplantation and humoral immunodeficiencies.
6.9.11.1. HIV-positive/AIDS patients
The first line treatment of sinusitis in HIV-positive patients is medical, in refractory cases targeted to the identified organisms. Surgical treatment is reserved for patients who do not respond to targeted medical treatment. Sabini and co-authors retrospectively reviewed their experience with performing endoscopic sinus surgery in 16 acquired immune deficiency (AIDS) patients (563). At an average follow-up time of 16 months, 14 of the endoscopic sinus surgery patients reported improvement from their preoperative condition (Evidence level IV). In a retrospective case series study, 106 HIV+ patients who underwent sinus surgery between 1987 and 1998 were evaluated (1949). Between 1987 and 1991, 36 patients were treated with minimal invasive sinus surgery just addressing the involved sinus with only 20% clinical improvement. Since 1992, the authors treated their HIV+ patients with more extensive surgery including sphenoethmoidectomy, middle meatal antrostomy and drainage of the frontal recess, which resulted in a clinical improvement rate of 75%, irrespective of the CD4 counts (Evidence level IV). In two case series, Murphy and co-workers observed the clinical outcome of 30 HIV-positive CRS patients refractory to medical treatment (1950). Outcome parameters included olfactory tests, symptom scores, and a quality of wellbeing assessment. Symptom and well-being scores improved significantly following endoscopic sinus surgery, whereas olfactory thresholds did not improve significantly (Evidence level IV). Patients with AIDS may develop acute invasive fungal sinusitis. If detected early, combined surgical and antifungal treatment may be beneficial (1951, 1952).
6.9.11.2. Bone marrow transplant
Bone marrow transplantation (BMT) is a frequent cause of acquired immune deficiency. Allogeneic BMT is associated with acute and chronic CRS in approximately 40% (1953). Sinus microbiology was investigated in 18 BMT patients who developed sinusitis evaluating 41 microbiological specimens obtained by antral puncture and nasal swabs from the middle meatus (1954). Agents most commonly isolated were gramnegative bacteria including Pseudomonas aeruginosa and Searratia marescens. Gram-positive bacteria were isolated in 27%. Various fungi were isolated in 16% of the specimens.
Microbiological results of antral punctures and nasal swabs were consistent in 5 of 41 specimens. Kennedy and co-workers report on 29 bone marrow transplant recipients with documented invasive fungal infections of the sinuses and paranasal tissues (1.7% of 1,692 bone marrow transplants performed). All patients received medical management, such as amphotericin, rifampin, and colony-stimulating factors, in addition to surgical intervention (1955). Surgical management ranged from minimally invasive procedures to extensive resections including medial maxillectomies. The mortality rate from the initial fungal infection was 62%. Twenty-seven percent resolved the initial infections but subsequently died of other causes. Prognosis was poor when cranial and orbital involvement and/or bony erosion occurred. Extensive surgery was not superior to endoscopic functional surgery (Evidence level IV). Sinus surgery was performed in 28 of 311 bone marrow trans-plant patients retrospectively evaluated (1956). No fungal sinusitis was observed. An aggressive surgical approach yielded a high mortality rate whereas limited surgical approaches with intensive postoperative care proved appropriate (Evidence level IV).
6.9.11.3. Non-acquired immunodeficiencies
Patients with humoral immunodeficiencies including common variable immunodeficiency, ataxia telangiectasia, or X-linked agammaglobulinaemia are at increased risk to develop CRS (1543, 1957-1959). Chee and co-workers selected 79 out of 316 patients with CRS with and without polyps, who suffered from severe CRS refractory to medical treatment (560). Fifty-seven patients had undergone one or more previous sinus surgeries. Approximately 30% of the 79 included patients suffered from decreased T-cell function and approximately 20% had some form of immunoglobulin deficiency. Common variable immunodeficiency was diagnosed in 10%. Accordingly, in a high number of patients with long lasting rhinosinusitis, humoral deficiencies were identified, particularly of the IgG3-subclass (1633, 1960). Also Carr et al. showed that patients with medically refractory CRS may have a high prevalence of lower serum IgA levels, low pre-immunization anti-pneumococcal titres and specific antibody deficiency (1533).
However, in unselected patients with sinus fungus ball, CRS with and without polyps, humoral deficiencies were not more frequent than in the general population (1961). Recently, the relevance of isolated immunoglobulin or IgG subclass deficiencies has been challenged and vaccine response to protein and capsular polysaccharides has been suggested superior to assess humoral immune function in CRS patients (1962 , 1963 , 1964 , 1965). Surgical outcome in patients with immunodeficiencies seems comparable to other CRS patients (1544, 1966).
Conclusion
In the small series available in HIV-positive patients, patients with bone marrow transplantation and patients with nonacquired immunodeficiencies endoscopic sinus surgery seems to be effective. In bone marrow transplantation patients with (fungal) infections extensive surgery was not superior to FESS. In non-acquired immunodeficiencies surgical outcomes are comparable to other CRS patients.
6.9.12. Fatigue
Fatigue is a common symptom in patients with CRS (75) and is associated with severity scores approximating those of facial pressure, headache, and nasal discharge. In a metaanalysis measuring the effect of FESS on fatigue, significant improvement in fatigue was noted equalling the improvement of pooled major CRS criteria (1967). In a study with subgroup analysis of 11 independent studies measured the response of fatigue following FESS in various groups, patients with more severe fatigue showed more pronounced improvement than patients with less severe fatigue (1968). Preoperative fatigue severity was less in patients with CRS and nasal polyposis than in patients with CRS only; however, preoperative fatigue was more severe in patients with fibromyalgia or depression.
6.9.13. Fibromyalgia
Patients with CRS and comorbid fibromyalgia showed similar improvements in QoL after FESS when compared with patients without fibromyalgia when controlling for age, gender, and disease severity (1969).
6.9.14. Biofilm
Bacterial biofilm formation was shown to be significantly associated with positive culture results, prior sinus surgeries, and nasal steroid use in the month prior to sample collection but not significantly associated with polyps, allergy, Samter's triad, sleep apnea, smoking status, age, or gender (1970). Different biofilm species are associated with different disease phenotypes. H. influenzae biofilms are typically found in patients with mild disease, whereas S. aureus is associated with a more severe, surgically recalcitrant pattern (692). Patients with biofilms have more severe disease preoperatively and persistence of postoperative symptoms, ongoing mucosal inflammation, and infections (686, 693, 1923, 1971). Asthma and biofilm-forming bacteria were shown to be independently associated with revision sinus surgeries for chronic rhinosinusitis (1923).
6.9.15. Smoking
The effect of smoking on outcome of FESS is unclear. Most studies show no effect of smoking on FESS outcomes (770, 1972-1974). Although one of the studies suggest that increased smoking may contribute to worse post-operative endoscopy scores (770). Another study showed that while smoking did not influence preoperative symptoms, smokers had worse postoperative outcomes (763)
6.9.16. Occupational exposure
It is known that airway exposure to occupational agents can give rise to occupational airway disease (1975). It was recently shown that exposure at work also appears to be a risk factor for the occurrence of CRS and for its recurrence or persistence, as evidenced by the need for revision surgery (1976).
6.9.17. Gastro-oesophageal reflux
Chambers et al (1421) showed in one hundred eighty-two patients that only gastro-oesophageal reflux disease was statistically significant as a predictor of poor symptomatic outcome. However, a number of other studies have failed to replicate this finding and it is likely that gastro-oesophageal reflux can mimic the symptoms of CRS rather that contribute to it (1788).
6.9.18. Osteitis
In a recent retrospective study the grade of osteitis was directly correlated with the number of revision surgeries, with an almost linear response. However, from the nature of the study it could not be clear if that was a cause-effect or a secondary association (1388). A study assessing the correlation between postoperative outcome and osteitis showed similar results, adding to the evidence for a link (1778) (Evidence level IV).
Immune deficiency states are frequently associated with CRS include HIV-infection, bone marrow transplantation and humoral immunodeficiencies.
6.9.11.1. HIV-positive/AIDS patients
The first line treatment of sinusitis in HIV-positive patients is medical, in refractory cases targeted to the identified organisms. Surgical treatment is reserved for patients who do not respond to targeted medical treatment. Sabini and co-authors retrospectively reviewed their experience with performing endoscopic sinus surgery in 16 acquired immune deficiency (AIDS) patients (563). At an average follow-up time of 16 months, 14 of the endoscopic sinus surgery patients reported improvement from their preoperative condition (Evidence level IV). In a retrospective case series study, 106 HIV+ patients who underwent sinus surgery between 1987 and 1998 were evaluated (1949). Between 1987 and 1991, 36 patients were treated with minimal invasive sinus surgery just addressing the involved sinus with only 20% clinical improvement. Since 1992, the authors treated their HIV+ patients with more extensive surgery including sphenoethmoidectomy, middle meatal antrostomy and drainage of the frontal recess, which resulted in a clinical improvement rate of 75%, irrespective of the CD4 counts (Evidence level IV). In two case series, Murphy and co-workers observed the clinical outcome of 30 HIV-positive CRS patients refractory to medical treatment (1950). Outcome parameters included olfactory tests, symptom scores, and a quality of wellbeing assessment. Symptom and well-being scores improved significantly following endoscopic sinus surgery, whereas olfactory thresholds did not improve significantly (Evidence level IV). Patients with AIDS may develop acute invasive fungal sinusitis. If detected early, combined surgical and antifungal treatment may be beneficial (1951, 1952).
6.9.11.2. Bone marrow transplant
Bone marrow transplantation (BMT) is a frequent cause of acquired immune deficiency. Allogeneic BMT is associated with acute and chronic CRS in approximately 40% (1953). Sinus microbiology was investigated in 18 BMT patients who developed sinusitis evaluating 41 microbiological specimens obtained by antral puncture and nasal swabs from the middle meatus (1954). Agents most commonly isolated were gramnegative bacteria including Pseudomonas aeruginosa and Searratia marescens. Gram-positive bacteria were isolated in 27%. Various fungi were isolated in 16% of the specimens.
Microbiological results of antral punctures and nasal swabs were consistent in 5 of 41 specimens. Kennedy and co-workers report on 29 bone marrow transplant recipients with documented invasive fungal infections of the sinuses and paranasal tissues (1.7% of 1,692 bone marrow transplants performed). All patients received medical management, such as amphotericin, rifampin, and colony-stimulating factors, in addition to surgical intervention (1955). Surgical management ranged from minimally invasive procedures to extensive resections including medial maxillectomies. The mortality rate from the initial fungal infection was 62%. Twenty-seven percent resolved the initial infections but subsequently died of other causes. Prognosis was poor when cranial and orbital involvement and/or bony erosion occurred. Extensive surgery was not superior to endoscopic functional surgery (Evidence level IV). Sinus surgery was performed in 28 of 311 bone marrow trans-plant patients retrospectively evaluated (1956). No fungal sinusitis was observed. An aggressive surgical approach yielded a high mortality rate whereas limited surgical approaches with intensive postoperative care proved appropriate (Evidence level IV).
6.9.11.3. Non-acquired immunodeficiencies
Patients with humoral immunodeficiencies including common variable immunodeficiency, ataxia telangiectasia, or X-linked agammaglobulinaemia are at increased risk to develop CRS (1543, 1957-1959). Chee and co-workers selected 79 out of 316 patients with CRS with and without polyps, who suffered from severe CRS refractory to medical treatment (560). Fifty-seven patients had undergone one or more previous sinus surgeries. Approximately 30% of the 79 included patients suffered from decreased T-cell function and approximately 20% had some form of immunoglobulin deficiency. Common variable immunodeficiency was diagnosed in 10%. Accordingly, in a high number of patients with long lasting rhinosinusitis, humoral deficiencies were identified, particularly of the IgG3-subclass (1633, 1960). Also Carr et al. showed that patients with medically refractory CRS may have a high prevalence of lower serum IgA levels, low pre-immunization anti-pneumococcal titres and specific antibody deficiency (1533).
However, in unselected patients with sinus fungus ball, CRS with and without polyps, humoral deficiencies were not more frequent than in the general population (1961). Recently, the relevance of isolated immunoglobulin or IgG subclass deficiencies has been challenged and vaccine response to protein and capsular polysaccharides has been suggested superior to assess humoral immune function in CRS patients (1962 , 1963 , 1964 , 1965). Surgical outcome in patients with immunodeficiencies seems comparable to other CRS patients (1544, 1966).
Conclusion
In the small series available in HIV-positive patients, patients with bone marrow transplantation and patients with nonacquired immunodeficiencies endoscopic sinus surgery seems to be effective. In bone marrow transplantation patients with (fungal) infections extensive surgery was not superior to FESS. In non-acquired immunodeficiencies surgical outcomes are comparable to other CRS patients.
6.9.12. Fatigue
Fatigue is a common symptom in patients with CRS (75) and is associated with severity scores approximating those of facial pressure, headache, and nasal discharge. In a metaanalysis measuring the effect of FESS on fatigue, significant improvement in fatigue was noted equalling the improvement of pooled major CRS criteria (1967). In a study with subgroup analysis of 11 independent studies measured the response of fatigue following FESS in various groups, patients with more severe fatigue showed more pronounced improvement than patients with less severe fatigue (1968). Preoperative fatigue severity was less in patients with CRS and nasal polyposis than in patients with CRS only; however, preoperative fatigue was more severe in patients with fibromyalgia or depression.
6.9.13. Fibromyalgia
Patients with CRS and comorbid fibromyalgia showed similar improvements in QoL after FESS when compared with patients without fibromyalgia when controlling for age, gender, and disease severity (1969).
6.9.14. Biofilm
Bacterial biofilm formation was shown to be significantly associated with positive culture results, prior sinus surgeries, and nasal steroid use in the month prior to sample collection but not significantly associated with polyps, allergy, Samter's triad, sleep apnea, smoking status, age, or gender (1970). Different biofilm species are associated with different disease phenotypes. H. influenzae biofilms are typically found in patients with mild disease, whereas S. aureus is associated with a more severe, surgically recalcitrant pattern (692). Patients with biofilms have more severe disease preoperatively and persistence of postoperative symptoms, ongoing mucosal inflammation, and infections (686, 693, 1923, 1971). Asthma and biofilm-forming bacteria were shown to be independently associated with revision sinus surgeries for chronic rhinosinusitis (1923).
6.9.15. Smoking
The effect of smoking on outcome of FESS is unclear. Most studies show no effect of smoking on FESS outcomes (770, 1972-1974). Although one of the studies suggest that increased smoking may contribute to worse post-operative endoscopy scores (770). Another study showed that while smoking did not influence preoperative symptoms, smokers had worse postoperative outcomes (763)
6.9.16. Occupational exposure
It is known that airway exposure to occupational agents can give rise to occupational airway disease (1975). It was recently shown that exposure at work also appears to be a risk factor for the occurrence of CRS and for its recurrence or persistence, as evidenced by the need for revision surgery (1976).
6.9.17. Gastro-oesophageal reflux
Chambers et al (1421) showed in one hundred eighty-two patients that only gastro-oesophageal reflux disease was statistically significant as a predictor of poor symptomatic outcome. However, a number of other studies have failed to replicate this finding and it is likely that gastro-oesophageal reflux can mimic the symptoms of CRS rather that contribute to it (1788).
6.9.18. Osteitis
In a recent retrospective study the grade of osteitis was directly correlated with the number of revision surgeries, with an almost linear response. However, from the nature of the study it could not be clear if that was a cause-effect or a secondary association (1388). A study assessing the correlation between postoperative outcome and osteitis showed similar results, adding to the evidence for a link (1778) (Evidence level IV).
6.10. Management of Paediatric Chronic Rhinosinusitis
6.10.1. Summary
CRS in children is not as well studied as the same entity in adults. Multiple factors contribute to the disease including bacteriologic and inflammatory factors. The adenoids are a prominent contributor to this entity in the paediatric age group. The mainstay of therapy is medical with surgical therapy reserved for the minority of patients who do not respond to medical treatment.
6.10.2. Medical treatment of chronic rhinosinusitis in children
6.10.2.1. antibiotics
There is no good evidence in the literature to support the use of antibiotics in CRS in children. Otten and colleagues investigated 141 children between the ages of 3 and 10 years with CRS as defined by purulent nasal drainage lasting at least 3 months, signs of purulent rhinitis on rhinoscopy, and unilateral or bilateral abnormalities of the maxillary sinus on plain films (1977). The patients were assigned non-selectively to receive one of the following 4 treatments for 10 days: saline nose drops (placebo), xylometazoline 0.5% nose drops with amoxicillin 250 mg PO TID, drainage of the maxillary sinus under anaesthesia and irrigation via indwelling catheter for at least 5 days, and a combination of drainage and irrigation with xylometazoline and amoxicillin. They followed the patients for up to 26 weeks after treatment and show no significant differences in cure rate among the treatments based on history, physical exam or maxillary sinus films. In the total group, the cure rate was around 69%. Although this study did not show a significant difference between the treatments, it suffers from some methodological limitations including lack of randomization or blinding, and that the placebo group actually received saline drops which might have been helpful in and of themselves. Further, this study does not assess the state of the ethmoid sinuses and used plain X-rays as the objective diagnostic modality. In a later study, the same group performed a randomized, double-blind study of cefaclor (20 mg/kg/day) vs placebo in 79 healthy children between the ages of 2 and 12 years with chronic sinusitis defined essentially as in the first study (1978). All patients had a tap and washout and were then randomized to cefaclor or placebo PO for 1 week and were followed at 6 weeks. After 6 weeks, there was no significant difference in resolution rate between the children on cefaclor (64.8%) and those on placebo (52.5%). Among the limitations of this study which could have influenced the outcome is that all children had an initial tap and washout which could have helped the whole group even before enrolment, making the antibiotic irrelevant, and plain radiographs were used to evaluate the sinuses.
Despite the lack of good evidence to support the use of antibiotics for any length of time in children with CRS, in practice, these children are often treated with the same antibiotics listed in the section on acute rhinosinusitis but typically for longer periods of time that vary between 3 and 6 weeks. Because of the lack of data to support this practice, its usefulness must be weighed against the increasing risks of inducing antimicrobial resistance. It is also difficult to ascertain whether what is actually being treated is CRS or acute exacerbations on top of pre-existing chronic disease. The exact type of antibiotics used is usually dependent on local resistance patterns which might be different in different countries. Further, it is advisable to always treat with as narrow a spectrum of antibiotics as will likely cover the bacteria that are prevalent in a specific geographic locale.
In sum, available data does not justify the use of short-term oral antibiotics for the treatment of CRS in children (Strength of recommendation: B). There might a place for longer-term antibiotics for the treatment of CRS in children (equivalent to CRS in adults) (Strength of recommendation: D).
Intravenous antibiotic therapy for CRS resistant to maximal medical treatment has been studied as an alternative to endoscopic sinus surgery. In a retrospective analysis of 70 children aged 10 months to 15 years with CRS, Don et al found that 89% had complete resolution of symptoms after maxillary sinus irrigation and selective adenoidectomy followed by one to 4 weeks of culture-directed intravenous antibiotics (1979). Cefuroxime IV was most frequently used followed by ampicillinsulbactam, ticarcillin clavulanate and vancomycin. Despite the good success rate, the therapy was not without adverse effects which included superficial thrombophlebtitis (9%), dislodgment of wire during placement necessitating venotomy (1%), and antibiotic related complications such as serum sickness, pseudomembranous colitis, and drug fevers. A similar retrospective study evaluated 22 children with CRS refractory to medical therapy and with an age range between 1.25 to14.5 years (1980). They all underwent adenoidectomy, maxillary sinus aspiration and irrigation and placement of intravenous catheters and then culture-directed IV antibiotic therapy until resolution of symptoms (mean duration of therapy was 5 weeks). All patients achieved control of symptoms at the end of IV therapy and 89% demonstrated long term amelioration of CRS symptoms (>12 months after cessation of IV therapy). The retrospective design, lack of randomization, and lack of placebo arms limit the value of the above studies. Furthermore, it is hard to assign benefit to intravenous antibiotic therapy when other interventions were utilized such as irrigation/aspiration of the sinus and adenoidectomy. Therefore available data does not justify the use of intravenous antibiotics alone for the treatment of CRS in children (Strength of recommendation: C).
6.10.2.2. Corticosteroids
There are no randomized controlled trials evaluating the effect of intranasal corticosteroids in children with CRS. However the combination of proven efficacy of intranasal corticosteroids in CRS with and without nasal polyps in adults (see chapter 6.1 and 6.5) and proven efficacy and safety of intranasal corticosteroids in allergic rhinitis in children makes intranasal corticosteroid the first line of treatment in CRS (1981 , 1982 , 1983). A recent randomized, placebo-controlled, double blind trial was conducted in children with CRS with signs and symptoms of more than 3 months duration and CT abnormalities (1984). Children were all treated with amoxicillin/clavulanate for 30 days and randomized to receive methylprednisolone or placebo PO for first 15 days of treatment (1mg/kg/day (max 40 mg) for 10 days, 0.75 mg/kg/ day for 2 days, 0.5 mg/kg/day for 2 days, and 0.25 mg/kg/day for 1 day). The average age of the children was 8 years and the total CT score was between 11-12 (maximal score=24) suggesting mild-moderate disease. When comparing post treatment outcomes to baseline, there were significant improvements in all parameters (symptoms and CT scores) in both groups suggesting that antibiotics alone and antibiotics and steroids together both improved outcomes compared to baseline. Furthermore, there was a significant additional effect of oral steroids over placebo in cough, CT scan, nasal obstruction, postnasal drainage and total symptom scores. The strength of the evidence for the efficacy of antibiotics alone is unfortunately diminished by the absence of a placebo group, but the superiority of the combination of antibiotics and steroids over antibiotics alone is clearly supported by this trial. Nasal corticosteroid treatment is a first line treatment in CRS with and without nasal polyps in children (Strength of recommendation: D).
6.10.2.3. Ancillary treatments
Nasal irrigations and decongestants have been thought to help in decreasing the frequency of rhinosinusitis episodes. Michel et al in 2005 performed a randomized, prospective, double-blind, controlled study looking at the effect of a 14-day treatment (1-2 sprays) with either isotonic saline solution or a nasal decongestant in children 2-6 years of age (1985). Outcomes evaluated included the degree of mucosal inflammation and nasal patency. They found that both groups experienced improvement in outcomes measured with no significant differences between the groups. There were no side effects observed with the saline spray. The decongestant group used 120% more drug than prescribed, demonstrating the potential for these medications to be overused. No cases of rhinitis medicamentosa were reported.
A recent Cochrane review analysed randomized controlled trials in which saline was evaluated in comparison with either no treatment, a placebo, as an adjunct to other treatments, or against other treatments (1736). A total of 8 trials satisfied inclusion criteria of which 3 were conducted in children. The studies included a broad range of delivery techniques, tonicity of saline used, and comparator treatments. Overall there was evidence that saline is beneficial in the treatment of the symptoms of CRS when used as the sole modality of treatment. Evidence also exists in favor of saline as a treatment adjunct and saline was not as effective as an intranasal steroid. Various forms of administration of saline were well tolerated. In a more recent trial, Wei and colleagues enrolled 40 children with CRS in a randomized, prospective, double-blind study comparing once daily irrigation with saline or saline/gentamicin for 6 weeks (1986). There were statistically significant improvements in quality of life scores after 3 weeks and a reduction of CT scores after 6 weeks in both groups with no significant difference between the groups, suggesting that the addition of gentamycin to saline irrigations provided no additional benefit.
Clinicians have certainly tried other treatments for CRS including antihistamines and leukotriene modifiers, especially in light of their effectiveness in treating allergic rhinitis. However no data exists about their potential efficacy and thus usefulness in the context of CRS in children. We reserve the use of these agents for children with documented allergic rhinitis.
6.10.1. Summary
CRS in children is not as well studied as the same entity in adults. Multiple factors contribute to the disease including bacteriologic and inflammatory factors. The adenoids are a prominent contributor to this entity in the paediatric age group. The mainstay of therapy is medical with surgical therapy reserved for the minority of patients who do not respond to medical treatment.
6.10.2. Medical treatment of chronic rhinosinusitis in children
6.10.2.1. antibiotics
There is no good evidence in the literature to support the use of antibiotics in CRS in children. Otten and colleagues investigated 141 children between the ages of 3 and 10 years with CRS as defined by purulent nasal drainage lasting at least 3 months, signs of purulent rhinitis on rhinoscopy, and unilateral or bilateral abnormalities of the maxillary sinus on plain films (1977). The patients were assigned non-selectively to receive one of the following 4 treatments for 10 days: saline nose drops (placebo), xylometazoline 0.5% nose drops with amoxicillin 250 mg PO TID, drainage of the maxillary sinus under anaesthesia and irrigation via indwelling catheter for at least 5 days, and a combination of drainage and irrigation with xylometazoline and amoxicillin. They followed the patients for up to 26 weeks after treatment and show no significant differences in cure rate among the treatments based on history, physical exam or maxillary sinus films. In the total group, the cure rate was around 69%. Although this study did not show a significant difference between the treatments, it suffers from some methodological limitations including lack of randomization or blinding, and that the placebo group actually received saline drops which might have been helpful in and of themselves. Further, this study does not assess the state of the ethmoid sinuses and used plain X-rays as the objective diagnostic modality. In a later study, the same group performed a randomized, double-blind study of cefaclor (20 mg/kg/day) vs placebo in 79 healthy children between the ages of 2 and 12 years with chronic sinusitis defined essentially as in the first study (1978). All patients had a tap and washout and were then randomized to cefaclor or placebo PO for 1 week and were followed at 6 weeks. After 6 weeks, there was no significant difference in resolution rate between the children on cefaclor (64.8%) and those on placebo (52.5%). Among the limitations of this study which could have influenced the outcome is that all children had an initial tap and washout which could have helped the whole group even before enrolment, making the antibiotic irrelevant, and plain radiographs were used to evaluate the sinuses.
Despite the lack of good evidence to support the use of antibiotics for any length of time in children with CRS, in practice, these children are often treated with the same antibiotics listed in the section on acute rhinosinusitis but typically for longer periods of time that vary between 3 and 6 weeks. Because of the lack of data to support this practice, its usefulness must be weighed against the increasing risks of inducing antimicrobial resistance. It is also difficult to ascertain whether what is actually being treated is CRS or acute exacerbations on top of pre-existing chronic disease. The exact type of antibiotics used is usually dependent on local resistance patterns which might be different in different countries. Further, it is advisable to always treat with as narrow a spectrum of antibiotics as will likely cover the bacteria that are prevalent in a specific geographic locale.
In sum, available data does not justify the use of short-term oral antibiotics for the treatment of CRS in children (Strength of recommendation: B). There might a place for longer-term antibiotics for the treatment of CRS in children (equivalent to CRS in adults) (Strength of recommendation: D).
Intravenous antibiotic therapy for CRS resistant to maximal medical treatment has been studied as an alternative to endoscopic sinus surgery. In a retrospective analysis of 70 children aged 10 months to 15 years with CRS, Don et al found that 89% had complete resolution of symptoms after maxillary sinus irrigation and selective adenoidectomy followed by one to 4 weeks of culture-directed intravenous antibiotics (1979). Cefuroxime IV was most frequently used followed by ampicillinsulbactam, ticarcillin clavulanate and vancomycin. Despite the good success rate, the therapy was not without adverse effects which included superficial thrombophlebtitis (9%), dislodgment of wire during placement necessitating venotomy (1%), and antibiotic related complications such as serum sickness, pseudomembranous colitis, and drug fevers. A similar retrospective study evaluated 22 children with CRS refractory to medical therapy and with an age range between 1.25 to14.5 years (1980). They all underwent adenoidectomy, maxillary sinus aspiration and irrigation and placement of intravenous catheters and then culture-directed IV antibiotic therapy until resolution of symptoms (mean duration of therapy was 5 weeks). All patients achieved control of symptoms at the end of IV therapy and 89% demonstrated long term amelioration of CRS symptoms (>12 months after cessation of IV therapy). The retrospective design, lack of randomization, and lack of placebo arms limit the value of the above studies. Furthermore, it is hard to assign benefit to intravenous antibiotic therapy when other interventions were utilized such as irrigation/aspiration of the sinus and adenoidectomy. Therefore available data does not justify the use of intravenous antibiotics alone for the treatment of CRS in children (Strength of recommendation: C).
6.10.2.2. Corticosteroids
There are no randomized controlled trials evaluating the effect of intranasal corticosteroids in children with CRS. However the combination of proven efficacy of intranasal corticosteroids in CRS with and without nasal polyps in adults (see chapter 6.1 and 6.5) and proven efficacy and safety of intranasal corticosteroids in allergic rhinitis in children makes intranasal corticosteroid the first line of treatment in CRS (1981 , 1982 , 1983). A recent randomized, placebo-controlled, double blind trial was conducted in children with CRS with signs and symptoms of more than 3 months duration and CT abnormalities (1984). Children were all treated with amoxicillin/clavulanate for 30 days and randomized to receive methylprednisolone or placebo PO for first 15 days of treatment (1mg/kg/day (max 40 mg) for 10 days, 0.75 mg/kg/ day for 2 days, 0.5 mg/kg/day for 2 days, and 0.25 mg/kg/day for 1 day). The average age of the children was 8 years and the total CT score was between 11-12 (maximal score=24) suggesting mild-moderate disease. When comparing post treatment outcomes to baseline, there were significant improvements in all parameters (symptoms and CT scores) in both groups suggesting that antibiotics alone and antibiotics and steroids together both improved outcomes compared to baseline. Furthermore, there was a significant additional effect of oral steroids over placebo in cough, CT scan, nasal obstruction, postnasal drainage and total symptom scores. The strength of the evidence for the efficacy of antibiotics alone is unfortunately diminished by the absence of a placebo group, but the superiority of the combination of antibiotics and steroids over antibiotics alone is clearly supported by this trial. Nasal corticosteroid treatment is a first line treatment in CRS with and without nasal polyps in children (Strength of recommendation: D).
6.10.2.3. Ancillary treatments
Nasal irrigations and decongestants have been thought to help in decreasing the frequency of rhinosinusitis episodes. Michel et al in 2005 performed a randomized, prospective, double-blind, controlled study looking at the effect of a 14-day treatment (1-2 sprays) with either isotonic saline solution or a nasal decongestant in children 2-6 years of age (1985). Outcomes evaluated included the degree of mucosal inflammation and nasal patency. They found that both groups experienced improvement in outcomes measured with no significant differences between the groups. There were no side effects observed with the saline spray. The decongestant group used 120% more drug than prescribed, demonstrating the potential for these medications to be overused. No cases of rhinitis medicamentosa were reported.
A recent Cochrane review analysed randomized controlled trials in which saline was evaluated in comparison with either no treatment, a placebo, as an adjunct to other treatments, or against other treatments (1736). A total of 8 trials satisfied inclusion criteria of which 3 were conducted in children. The studies included a broad range of delivery techniques, tonicity of saline used, and comparator treatments. Overall there was evidence that saline is beneficial in the treatment of the symptoms of CRS when used as the sole modality of treatment. Evidence also exists in favor of saline as a treatment adjunct and saline was not as effective as an intranasal steroid. Various forms of administration of saline were well tolerated. In a more recent trial, Wei and colleagues enrolled 40 children with CRS in a randomized, prospective, double-blind study comparing once daily irrigation with saline or saline/gentamicin for 6 weeks (1986). There were statistically significant improvements in quality of life scores after 3 weeks and a reduction of CT scores after 6 weeks in both groups with no significant difference between the groups, suggesting that the addition of gentamycin to saline irrigations provided no additional benefit.
Clinicians have certainly tried other treatments for CRS including antihistamines and leukotriene modifiers, especially in light of their effectiveness in treating allergic rhinitis. However no data exists about their potential efficacy and thus usefulness in the context of CRS in children. We reserve the use of these agents for children with documented allergic rhinitis.
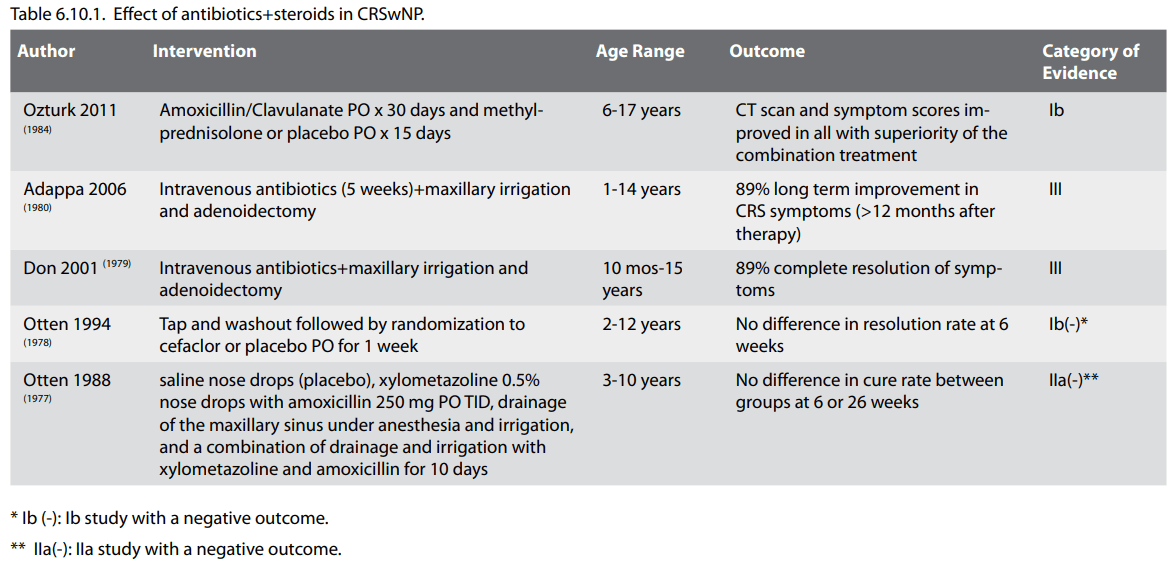
6.10.3. Surgical treatment of chronic rhinosinusitis in
children
Adenoidectomy is successful in improving CRS symptoms in 50% of operated children. Whether this is due to the fact that the symptoms were related to adenoiditis per se or to the elimination of the contribution of the adenoids to sinus disease is not clear
Surgical intervention for rhinosinusitis is usually considered for patients with CRS who have failed maximal medical therapy. This is hard to define but usually includes a course of antibiotics and intranasal and/or systemic steroids and differs widely between practitioners and practice locations. Adenoidectomy with or without antral irrigation and balloon sinus dilation, and functional endoscopic sinus surgery (FESS) are the most commonly used modalities.
6.10.3.1 Adenoidectomy with/without sinus irrigation and balloon dilation
The rationale behind removal of the adenoids in patients with CRS stems from the hypothesis that the adenoids are a nasopharyngeal bacterial reservoir (as detailed earlier) and the possibility that many of the symptoms might be related to adenoiditis proper. The benefit of adenoidectomy alone in the treatment of children with CRS was recently evaluated by a meta-analysis (1987). The review included 9 studies that met the inclusion criteria. Mean sample size was 46 subjects with a mean age of 5.8 years (range 4.4-6.9 years). All studies showed that sinusitis symptoms or outcomes improved in half or more patients after adenoidectomy. Eight of nine studies were sufficiently similar to undergo meta-analysis and, in these, the summary estimate of the proportion of patients who significantly improved after adenoidectomy was 69.3%. Ramadan and Tiu reported on the failures of adenoidectomy over a ten year period and found that children younger than 7 years of age and those with asthma were more likely to fail after adenoidectomy and go on to require salvage FESS (1988). Maxillary antral irrigation is frequently performed in conjunction with adenoidectomy. To evaluate the efficacy of this added intervention, Ramadan and colleagues analysed 60 children who underwent adenoidectomy for CRS (symptoms and positive scans despite prolonged medical treatment), 32 of which also had a sinus wash and culture via the middle meatus (1989). All children received post-operative antibiotics for 2 weeks and outcomes were assessed at least 12 months postoperatively. Patients who underwent adenoidectomy alone had a 61% success rate at 12 months compared to children who underwent adenoidectomy with a sinus wash who had a higher success rate of 88%. Children with a high Lund-Mackay CT score and asthma had better success with adenoidectomy with a wash compared to adenoidectomy alone. In a similar retrospective study, Criddle and colleagues reviewed the records of 23 children who had adenoidectomy with a sinus wash for CRS (persistent symptoms in all and a positive scan in 7/23) followed by a course of post-op oral antibiotics (average duration 5.8 weeks) (1990). If there was no improvement after the procedure on oral antibiotics, intravenous antibiotics were utilized in a small proportion of the children. Long-term resolution rate was reported in 78% of the 18 patients who did not need intravenous antibiotics. This data suggests that antral irrigation adds to the efficacy of adenoidectomy and also suggests that a prolonged course of IV antibiotics (as reported above) might not be necessary to obtain a good result.
Balloon sinuplasty was approved by the FDA for use in children in the United States in 2006, and a preliminary study in children has shown the procedure to be safe and feasible (1991). In this study, the cannulation success rate was 91% and the majority of the sinuses addressed were maxillaries. The most common cause of failure of cannulation with the balloon catheter was the presence of a hypoplastic maxillary sinus. Most surgeons now use the illuminated catheter to confirm cannulation of the sinus thus avoiding fluoroscopy and its inherent risks. In a recent nonrandomized, prospective evaluation of children with CRS failing maximal medical therapy, balloon catheter sinuplasty and adenoidectomy were compared (1992). Outcomes were assessed at 1 year after surgery and were based on SN-5 scores and the need for revision surgery. Twenty four/30 patients (80%) who underwent balloon sinuplasty showed improvement in their symptoms compared to 10/19 (52.6%) of the patients who underwent adenoidectomy (p<0.05). As some of the balloon patients also underwent irrigation, it is hard to discern the effect of dilation vs irrigation from this study. In sum, most of the available surgical data support adenoidectomy with sinus irrigation as a first step in the management of the child with CRS refractory to maximal medical management. Whether or not balloon maxillary sinuplasty imparts additional benefit to irrigation alone, in combination with adenoidectomy, cannot be established with available data to date (Strength of recommendation: C).
Adenoidectomy is successful in improving CRS symptoms in 50% of operated children. Whether this is due to the fact that the symptoms were related to adenoiditis per se or to the elimination of the contribution of the adenoids to sinus disease is not clear
Surgical intervention for rhinosinusitis is usually considered for patients with CRS who have failed maximal medical therapy. This is hard to define but usually includes a course of antibiotics and intranasal and/or systemic steroids and differs widely between practitioners and practice locations. Adenoidectomy with or without antral irrigation and balloon sinus dilation, and functional endoscopic sinus surgery (FESS) are the most commonly used modalities.
6.10.3.1 Adenoidectomy with/without sinus irrigation and balloon dilation
The rationale behind removal of the adenoids in patients with CRS stems from the hypothesis that the adenoids are a nasopharyngeal bacterial reservoir (as detailed earlier) and the possibility that many of the symptoms might be related to adenoiditis proper. The benefit of adenoidectomy alone in the treatment of children with CRS was recently evaluated by a meta-analysis (1987). The review included 9 studies that met the inclusion criteria. Mean sample size was 46 subjects with a mean age of 5.8 years (range 4.4-6.9 years). All studies showed that sinusitis symptoms or outcomes improved in half or more patients after adenoidectomy. Eight of nine studies were sufficiently similar to undergo meta-analysis and, in these, the summary estimate of the proportion of patients who significantly improved after adenoidectomy was 69.3%. Ramadan and Tiu reported on the failures of adenoidectomy over a ten year period and found that children younger than 7 years of age and those with asthma were more likely to fail after adenoidectomy and go on to require salvage FESS (1988). Maxillary antral irrigation is frequently performed in conjunction with adenoidectomy. To evaluate the efficacy of this added intervention, Ramadan and colleagues analysed 60 children who underwent adenoidectomy for CRS (symptoms and positive scans despite prolonged medical treatment), 32 of which also had a sinus wash and culture via the middle meatus (1989). All children received post-operative antibiotics for 2 weeks and outcomes were assessed at least 12 months postoperatively. Patients who underwent adenoidectomy alone had a 61% success rate at 12 months compared to children who underwent adenoidectomy with a sinus wash who had a higher success rate of 88%. Children with a high Lund-Mackay CT score and asthma had better success with adenoidectomy with a wash compared to adenoidectomy alone. In a similar retrospective study, Criddle and colleagues reviewed the records of 23 children who had adenoidectomy with a sinus wash for CRS (persistent symptoms in all and a positive scan in 7/23) followed by a course of post-op oral antibiotics (average duration 5.8 weeks) (1990). If there was no improvement after the procedure on oral antibiotics, intravenous antibiotics were utilized in a small proportion of the children. Long-term resolution rate was reported in 78% of the 18 patients who did not need intravenous antibiotics. This data suggests that antral irrigation adds to the efficacy of adenoidectomy and also suggests that a prolonged course of IV antibiotics (as reported above) might not be necessary to obtain a good result.
Balloon sinuplasty was approved by the FDA for use in children in the United States in 2006, and a preliminary study in children has shown the procedure to be safe and feasible (1991). In this study, the cannulation success rate was 91% and the majority of the sinuses addressed were maxillaries. The most common cause of failure of cannulation with the balloon catheter was the presence of a hypoplastic maxillary sinus. Most surgeons now use the illuminated catheter to confirm cannulation of the sinus thus avoiding fluoroscopy and its inherent risks. In a recent nonrandomized, prospective evaluation of children with CRS failing maximal medical therapy, balloon catheter sinuplasty and adenoidectomy were compared (1992). Outcomes were assessed at 1 year after surgery and were based on SN-5 scores and the need for revision surgery. Twenty four/30 patients (80%) who underwent balloon sinuplasty showed improvement in their symptoms compared to 10/19 (52.6%) of the patients who underwent adenoidectomy (p<0.05). As some of the balloon patients also underwent irrigation, it is hard to discern the effect of dilation vs irrigation from this study. In sum, most of the available surgical data support adenoidectomy with sinus irrigation as a first step in the management of the child with CRS refractory to maximal medical management. Whether or not balloon maxillary sinuplasty imparts additional benefit to irrigation alone, in combination with adenoidectomy, cannot be established with available data to date (Strength of recommendation: C).
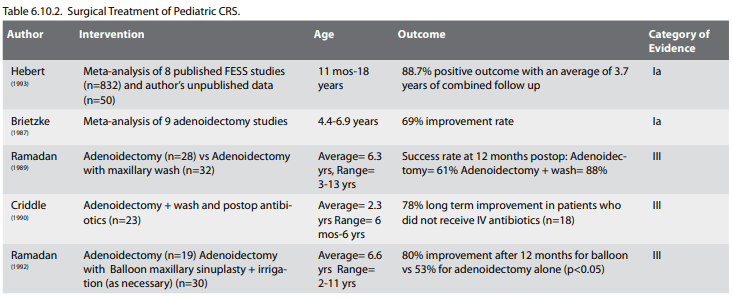
6.10.3.2. Functional Endoscopic Sinus Surgery (FESS)
A meta-analysis of FESS results in the paediatric population has shown that this surgical modality is effective in reducing symptoms with an 88% success rate and a low complication rate (1993). Initial concerns about possible adverse effects of FESS on facial growth have been allayed by a long term follow up study by Bothwell and colleagues that showed no impact of FESS on qualitative and quantitative parameters of paediatric facial growth, evaluated up to 10 years postoperatively (1994). Many advocate a limited approach to FESS in children consisting of removal of any obvious obstruction (such as polyps and concha bullosa), as well as anterior bulla ethmoidectomy and maxillary antrostomy. This approach typically yields significant improvements in nasal obstruction (91%), rhinorrhoea (90%), PND (90%), headache (97%), hyposmia (89%) and chronic cough (96%) (1995).
Whereas second look procedures were common after FESS to clean the cavities, the advent of absorbable packing has made it possible to avoid a second look procedure. Walner et al found comparable rates of revision sinus surgery in children with and without a second look procedure suggesting that it may not be necessary (1996). Ramadan and colleagues observed that the use of corticosteroids during initial FESS might obviate a second look procedure (1997). Younis in a review of available data suggested that a second look is not necessary in most children after FESS (1998).
There are few reports on the causes of failure of ESS in children. The most comprehensive describes 23 of 176 (13%) children who failed FESS and required revision (1991). The most common findings in these patients were adhesions (57%) and maxillary sinus ostium stenosis or missed maxillary sinus ostium (52%). In 39% of the cases, disease recurred in the operated sinuses, whereas in 26% of the cases, surgery was needed because of disease present in sinuses that were not originally operated. In another report, a retrospective review of children with CRS having undergone ESS yielded 39.6% who continued to have mucopurulent nasal drainage for more than 3 months after surgery (1999). Sinonasal polyposis, history of allergic rhinitis, and male gender were significantly more frequently observed in the group that continued to have problems after ESS.
In summary, the most supported surgical approach to the child with CRS who has failed maximal medical therapy probably consists of an initial attempt at an adenoidectomy with a maxillary sinus wash plus/minus balloon dilation followed by FESS in case of recurrence of symptoms. An exception to this statement are children with cystic fibrosis, nasal polyposis, antrochoanal polyposis, or AFS where FESS to decrease disease burden is the initial favoured surgical option. Unfortunately, most of the data supporting this recommendation are not based on randomized prospective studies. It is therefore clear that prospective, randomised, controlled clinical trials should be undertaken. In these trials, severity of disease on CT scans and symptom questionnaire should ideally be matched preoperatively and the following interventions would be compared: adenoidectomy alone, adenoidectomy with a wash, adenoidectomy with a wash and balloon maxillary sinuplasty, and endoscopic sinus surgery. An additional arm that includes medical therapy might also be included.
A meta-analysis of FESS results in the paediatric population has shown that this surgical modality is effective in reducing symptoms with an 88% success rate and a low complication rate (1993). Initial concerns about possible adverse effects of FESS on facial growth have been allayed by a long term follow up study by Bothwell and colleagues that showed no impact of FESS on qualitative and quantitative parameters of paediatric facial growth, evaluated up to 10 years postoperatively (1994). Many advocate a limited approach to FESS in children consisting of removal of any obvious obstruction (such as polyps and concha bullosa), as well as anterior bulla ethmoidectomy and maxillary antrostomy. This approach typically yields significant improvements in nasal obstruction (91%), rhinorrhoea (90%), PND (90%), headache (97%), hyposmia (89%) and chronic cough (96%) (1995).
Whereas second look procedures were common after FESS to clean the cavities, the advent of absorbable packing has made it possible to avoid a second look procedure. Walner et al found comparable rates of revision sinus surgery in children with and without a second look procedure suggesting that it may not be necessary (1996). Ramadan and colleagues observed that the use of corticosteroids during initial FESS might obviate a second look procedure (1997). Younis in a review of available data suggested that a second look is not necessary in most children after FESS (1998).
There are few reports on the causes of failure of ESS in children. The most comprehensive describes 23 of 176 (13%) children who failed FESS and required revision (1991). The most common findings in these patients were adhesions (57%) and maxillary sinus ostium stenosis or missed maxillary sinus ostium (52%). In 39% of the cases, disease recurred in the operated sinuses, whereas in 26% of the cases, surgery was needed because of disease present in sinuses that were not originally operated. In another report, a retrospective review of children with CRS having undergone ESS yielded 39.6% who continued to have mucopurulent nasal drainage for more than 3 months after surgery (1999). Sinonasal polyposis, history of allergic rhinitis, and male gender were significantly more frequently observed in the group that continued to have problems after ESS.
In summary, the most supported surgical approach to the child with CRS who has failed maximal medical therapy probably consists of an initial attempt at an adenoidectomy with a maxillary sinus wash plus/minus balloon dilation followed by FESS in case of recurrence of symptoms. An exception to this statement are children with cystic fibrosis, nasal polyposis, antrochoanal polyposis, or AFS where FESS to decrease disease burden is the initial favoured surgical option. Unfortunately, most of the data supporting this recommendation are not based on randomized prospective studies. It is therefore clear that prospective, randomised, controlled clinical trials should be undertaken. In these trials, severity of disease on CT scans and symptom questionnaire should ideally be matched preoperatively and the following interventions would be compared: adenoidectomy alone, adenoidectomy with a wash, adenoidectomy with a wash and balloon maxillary sinuplasty, and endoscopic sinus surgery. An additional arm that includes medical therapy might also be included.