Chronic Rhinosinusitis with or without nasal polyps
(CRSwNP or CRSsNP)
(CRSwNP or CRSsNP)
4.1. Epidemiology and predisposing factors
4.1.1. Summary
The overview of the currently available literature illustrates the paucity of accurate information on the epidemiology of CRSsNP and CRSwNP, especially in European countries, and highlights the need for large-scale epidemiologic research exploring their prevalence and incidence. Only by the use of well standardized definitions for CRSs and wNP, and well-defined inclusion criteria for epidemiologic research, will it be possible to obtain accurate epidemiologic data on the natural evolution of these diseases, the influence of ethnic background and genetic factors and the factors associated with the disease manifestation.
4.1.2. Introduction
Chronic rhinosinusitis with (CRSwNP) and without nasal polyps (CRSsNP) in its many forms, constitutes one of the commonest conditions encountered in medicine and may present to a wide range of clinicians from primary care to accident and emergency, pulmonologists, allergists, otorhinolaryngologists and even intensivists and neurosurgeons when severe complications occur (483).
4.1.3. Epidemiology of CRSwNP and CRSsNP.
There is a deficit of epidemiologic studies exploring the prevalence and incidence of CRSsNP and CRSwNP especially in European countries.
4.1.3.1. CRSsNP.
The paucity of accurate epidemiologic data on CRS contrasts with the more abundant information on microbiology, diagnosis and treatment options for these conditions. When reviewing the current literature on CRS, it becomes clear that giving an accurate estimate of the prevalence of CRS remains speculative, because of the heterogeneity of the disorder and the diagnostic imprecision often used in publications. In a survey on the prevalence of chronic conditions, it was estimated that CRS, defined as having 'sinus trouble' for more than 3 months in the year before the interview, affects 15.5% of the total population in the United States (484) ranking this condition second in prevalence among all chronic conditions. Subsequently the high prevalence of CRS was confirmed by another survey suggesting that 16% of the adult US population has CRS (485). However, the prevalence of doctor-diagnosed CRS is much lower; a prevalence of 2% was found using ICD-9 codes as an identifier (486). Corroboration of the definitive diagnosis of CRS should be done with nasal endoscopy (487) or CT (488) As the diagnosis of CRS has primarily been based on symptoms, often excluding dysosmia, this means that the diagnosis of CRS is often overestimated (11, 488). The majority of primary care physicians do not have the training or equipment to perform nasal endoscopy, which also leads to overdiagnosis (489). Interestingly, the prevalence rate of CRS was substantially higher in females with a female/male ratio of 6/4 (484). In Canada, the prevalence of CRS, defined as an affirmative answer to the question 'Has the patient had sinusitis diagnosed by a health professional lasting for more than 6 months?' ranged from 3.4% in male to 5.7% in female subjects (490). The prevalence increased with age, with a mean of 2.7% and 6.6% in the age groups of 20-29 and 50 59 years, respectively. After the age of 60 years, prevalence levels of CRS levelled off to 4.7% (490). In a nationwide survey in Korea, the overall prevalence of CRS, defined as the presence of at least 3 nasal symptoms lasting more than 3 months together with the endoscopic finding of nasal polyps and/or mucopurulent discharge within the middle meatus, was 1.01% (491), with no differences between age groups or gender. By screening a non-ENT population, which may be considered representative of the general population in Belgium, Gordts et al. (492) reported that 6% of subjects suffered from chronic nasal discharge. A comparative study in the north of Scotland and the Caribbean found that in ORL clinics in both populations there was a similar prevalence of CRS (9.6% and 9.3% respectively) (493).
Recently, a postal questionnaire on the EPOS criteria was sent to a random sample of adults aged 15-75 years in 19 centres in Europe. The Global Allergy and Asthma Network of Excellence (GA2LEN) study concluded that the overall prevalence of CRS by EP3 OS criteria was 10.9% (range 6.9- 27.1) (12). A very recent study in Sao Paulo using personal interviews and defining CRS based on the EPOS criteria found a prevalence of 5.5% (1368).
Recent data have demonstrated that CRS affects approximately 5–15% of the general population both in Europe and the USA. The prevalence of doctor-diagnosed CRS was 2-4%.
4.1.1. Summary
The overview of the currently available literature illustrates the paucity of accurate information on the epidemiology of CRSsNP and CRSwNP, especially in European countries, and highlights the need for large-scale epidemiologic research exploring their prevalence and incidence. Only by the use of well standardized definitions for CRSs and wNP, and well-defined inclusion criteria for epidemiologic research, will it be possible to obtain accurate epidemiologic data on the natural evolution of these diseases, the influence of ethnic background and genetic factors and the factors associated with the disease manifestation.
4.1.2. Introduction
Chronic rhinosinusitis with (CRSwNP) and without nasal polyps (CRSsNP) in its many forms, constitutes one of the commonest conditions encountered in medicine and may present to a wide range of clinicians from primary care to accident and emergency, pulmonologists, allergists, otorhinolaryngologists and even intensivists and neurosurgeons when severe complications occur (483).
4.1.3. Epidemiology of CRSwNP and CRSsNP.
There is a deficit of epidemiologic studies exploring the prevalence and incidence of CRSsNP and CRSwNP especially in European countries.
4.1.3.1. CRSsNP.
The paucity of accurate epidemiologic data on CRS contrasts with the more abundant information on microbiology, diagnosis and treatment options for these conditions. When reviewing the current literature on CRS, it becomes clear that giving an accurate estimate of the prevalence of CRS remains speculative, because of the heterogeneity of the disorder and the diagnostic imprecision often used in publications. In a survey on the prevalence of chronic conditions, it was estimated that CRS, defined as having 'sinus trouble' for more than 3 months in the year before the interview, affects 15.5% of the total population in the United States (484) ranking this condition second in prevalence among all chronic conditions. Subsequently the high prevalence of CRS was confirmed by another survey suggesting that 16% of the adult US population has CRS (485). However, the prevalence of doctor-diagnosed CRS is much lower; a prevalence of 2% was found using ICD-9 codes as an identifier (486). Corroboration of the definitive diagnosis of CRS should be done with nasal endoscopy (487) or CT (488) As the diagnosis of CRS has primarily been based on symptoms, often excluding dysosmia, this means that the diagnosis of CRS is often overestimated (11, 488). The majority of primary care physicians do not have the training or equipment to perform nasal endoscopy, which also leads to overdiagnosis (489). Interestingly, the prevalence rate of CRS was substantially higher in females with a female/male ratio of 6/4 (484). In Canada, the prevalence of CRS, defined as an affirmative answer to the question 'Has the patient had sinusitis diagnosed by a health professional lasting for more than 6 months?' ranged from 3.4% in male to 5.7% in female subjects (490). The prevalence increased with age, with a mean of 2.7% and 6.6% in the age groups of 20-29 and 50 59 years, respectively. After the age of 60 years, prevalence levels of CRS levelled off to 4.7% (490). In a nationwide survey in Korea, the overall prevalence of CRS, defined as the presence of at least 3 nasal symptoms lasting more than 3 months together with the endoscopic finding of nasal polyps and/or mucopurulent discharge within the middle meatus, was 1.01% (491), with no differences between age groups or gender. By screening a non-ENT population, which may be considered representative of the general population in Belgium, Gordts et al. (492) reported that 6% of subjects suffered from chronic nasal discharge. A comparative study in the north of Scotland and the Caribbean found that in ORL clinics in both populations there was a similar prevalence of CRS (9.6% and 9.3% respectively) (493).
Recently, a postal questionnaire on the EPOS criteria was sent to a random sample of adults aged 15-75 years in 19 centres in Europe. The Global Allergy and Asthma Network of Excellence (GA2LEN) study concluded that the overall prevalence of CRS by EP3 OS criteria was 10.9% (range 6.9- 27.1) (12). A very recent study in Sao Paulo using personal interviews and defining CRS based on the EPOS criteria found a prevalence of 5.5% (1368).
Recent data have demonstrated that CRS affects approximately 5–15% of the general population both in Europe and the USA. The prevalence of doctor-diagnosed CRS was 2-4%.
4.1.3.2. CRSwNP
Epidemiologic studies rely on nasal endoscopy and/or questionnaires to report on the prevalence of nasal polyps. Large NP can be visualized by anterior rhinoscopy, whereas nasal endoscopy is warranted for the diagnosis of smaller NP. Nasal endoscopy is, therefore, a prerequisite for an accurate estimate of the prevalence of NP, as not all patients that claim to have NP actually have polyps on nasal endoscopy (494). Thus, surveys based on questionnaires asking for the presence of NP, may provide us with an overestimation of the self-reported prevalence of NP. Recently, a French expert panel of ENT specialists elaborated a diagnostic questionnaire/algorithm with 90% sensitivity and specificity (495). In the light of epidemiologic research, a distinction needs to be made between clinically silent NP or preclinical cases, and symptomatic NP.
Asymptomatic polyps may transiently be present or persist, and hence remain undiagnosed until they are discovered by clinical examination. On the other hand, polyps that become symptomatic may remain undiagnosed, either because they are missed during anterior rhinoscopy and/or because patients do not see their doctor for this problem. Indeed, one third of patients with CRSwNP do not seek medical advice for their sinonasal symptoms (496). Compared to patients with CRSwNP not seeking medical attention, those actively seeking medical care for CRSwNP had more extensive NP with more reduction of peak nasal inspiratory flow and greater impairment of the sense of smell (497).
In a population-based study in Skovde, Sweden, Johansson et al. (494) reported a prevalence of nasal polyps of 2.7% of the total population. In this study, NP were diagnosed by nasal endoscopy and were more frequent in men (2.2 to 1), the elderly (5% at 60 years of age and older) and asthmatics. In a nationwide survey in Korea, the overall prevalence of polyps diagnosed by nasal endoscopy was 0.5% of the total population (498). Based on a postal questionnaire survey in Finland, Hedman et al. (499) found that 4.3% of the adult population answered positively to the question as to whether polyps had been found in their nose. Using a disease-specific questionnaire, Klossek et al. (496) reported a prevalence of NP of 2.1% in France. From autopsy studies, a prevalence of 2% has been found using anterior rhinoscopy (500). In Denmark after removing whole naso-ethmoidal blocks, nasal polyps were found in 5 of 19 cadavers (501). and in 42% of 31 autopsy samples combining endoscopy with endoscopic sinus surgery (502). The median age of the cases in the 3 autopsy studies by Larsen and Tos ranged from 70 to 79 years. From these cadaver studies, one may conclude that a significant number of patients with NP do not feel the need to seek medical attention or that the diagnosis of NP is often missed by doctors. It has been stated that between 0.2% and 1% of people develop NP at some stage (503). In a prospective study on the incidence of symptomatic NP, Larsen and Tos (504) found an estimated incidence of 0.86 and 0.39 patients per thousand per year for males and females, respectively. The incidence increased with age, reaching peaks of 1.68 and 0.82 patients per thousand per year for males and females respectively in the age group of 50-59 years. When reviewing data from patient records of nearly 5,000 patients from hospitals and allergy clinics in the US in 1977, the prevalence of NP was found to be 4.2% (505), with a higher prevalence (6.7%) in the asthmatic patients. In general, NPs occur in all races and becomes more common with age (496, 506-509). The average age of onset is approximately 42 years, which is 7 years older than the average age of the onset of asthma (510-512). NPs are uncommon under the age of 20 (513) and are more frequently found in men than in women (499, 504, 514), except in the studies by Settipane (505) and Klossek (496)
Szczeklik et al. (515) studied the natural history of asthma and CRS in 16 clinical centres in 10 European countries. Rhinitis was the first symptom of the disease. It appeared on average at an age of 30 yrs. It was perennial, difficult to treat and led to loss of smell in 55% of patients. In an average patient, 2 yrs alter commencement of rhinitis, the first symptoms of asthma appeared. Intolerance to aspirin and/or other NSAIDs became evident 4 yrs later. Nasal polyps were diagnosed at about the same time in 60% of subjects. There was a close linear association between mean age at onset of rhinitis, asthma, NSAID intolerance and nasal polyps (515).
Epidemiologic studies rely on nasal endoscopy and/or questionnaires to report on the prevalence of nasal polyps. Large NP can be visualized by anterior rhinoscopy, whereas nasal endoscopy is warranted for the diagnosis of smaller NP. Nasal endoscopy is, therefore, a prerequisite for an accurate estimate of the prevalence of NP, as not all patients that claim to have NP actually have polyps on nasal endoscopy (494). Thus, surveys based on questionnaires asking for the presence of NP, may provide us with an overestimation of the self-reported prevalence of NP. Recently, a French expert panel of ENT specialists elaborated a diagnostic questionnaire/algorithm with 90% sensitivity and specificity (495). In the light of epidemiologic research, a distinction needs to be made between clinically silent NP or preclinical cases, and symptomatic NP.
Asymptomatic polyps may transiently be present or persist, and hence remain undiagnosed until they are discovered by clinical examination. On the other hand, polyps that become symptomatic may remain undiagnosed, either because they are missed during anterior rhinoscopy and/or because patients do not see their doctor for this problem. Indeed, one third of patients with CRSwNP do not seek medical advice for their sinonasal symptoms (496). Compared to patients with CRSwNP not seeking medical attention, those actively seeking medical care for CRSwNP had more extensive NP with more reduction of peak nasal inspiratory flow and greater impairment of the sense of smell (497).
In a population-based study in Skovde, Sweden, Johansson et al. (494) reported a prevalence of nasal polyps of 2.7% of the total population. In this study, NP were diagnosed by nasal endoscopy and were more frequent in men (2.2 to 1), the elderly (5% at 60 years of age and older) and asthmatics. In a nationwide survey in Korea, the overall prevalence of polyps diagnosed by nasal endoscopy was 0.5% of the total population (498). Based on a postal questionnaire survey in Finland, Hedman et al. (499) found that 4.3% of the adult population answered positively to the question as to whether polyps had been found in their nose. Using a disease-specific questionnaire, Klossek et al. (496) reported a prevalence of NP of 2.1% in France. From autopsy studies, a prevalence of 2% has been found using anterior rhinoscopy (500). In Denmark after removing whole naso-ethmoidal blocks, nasal polyps were found in 5 of 19 cadavers (501). and in 42% of 31 autopsy samples combining endoscopy with endoscopic sinus surgery (502). The median age of the cases in the 3 autopsy studies by Larsen and Tos ranged from 70 to 79 years. From these cadaver studies, one may conclude that a significant number of patients with NP do not feel the need to seek medical attention or that the diagnosis of NP is often missed by doctors. It has been stated that between 0.2% and 1% of people develop NP at some stage (503). In a prospective study on the incidence of symptomatic NP, Larsen and Tos (504) found an estimated incidence of 0.86 and 0.39 patients per thousand per year for males and females, respectively. The incidence increased with age, reaching peaks of 1.68 and 0.82 patients per thousand per year for males and females respectively in the age group of 50-59 years. When reviewing data from patient records of nearly 5,000 patients from hospitals and allergy clinics in the US in 1977, the prevalence of NP was found to be 4.2% (505), with a higher prevalence (6.7%) in the asthmatic patients. In general, NPs occur in all races and becomes more common with age (496, 506-509). The average age of onset is approximately 42 years, which is 7 years older than the average age of the onset of asthma (510-512). NPs are uncommon under the age of 20 (513) and are more frequently found in men than in women (499, 504, 514), except in the studies by Settipane (505) and Klossek (496)
Szczeklik et al. (515) studied the natural history of asthma and CRS in 16 clinical centres in 10 European countries. Rhinitis was the first symptom of the disease. It appeared on average at an age of 30 yrs. It was perennial, difficult to treat and led to loss of smell in 55% of patients. In an average patient, 2 yrs alter commencement of rhinitis, the first symptoms of asthma appeared. Intolerance to aspirin and/or other NSAIDs became evident 4 yrs later. Nasal polyps were diagnosed at about the same time in 60% of subjects. There was a close linear association between mean age at onset of rhinitis, asthma, NSAID intolerance and nasal polyps (515).
4.1.4. Factors associated with CRSwNP and CRSsNP
4.1.4.1. Ciliary impairment
As may be concluded from the section on anatomy and pathophysiology, ciliary function plays an important role in the clearance of the sinuses and the prevention of chronic inflammation. Secondary ciliary dyskinesia is found in patients with CRS, and is probably reversible, although restoration takes some time (516). As expected in patients with Kartagener's syndrome and primary ciliary dyskinesia, CRS is a common problem and these patients usually have a long history of respiratory infections.
In patients with cystic fibrosis (CF), the inability of the cilia to transport the viscous mucus causes ciliary malfunction and consequently CRS. NPs are present in about 40% of patients with CF (517). These polyps are generally more neutrophilic than eosinophilic in nature.
4.1.4.2. Allergy
Review articles on CRS have suggested that atopy predisposes to its development (518 , 519). It is tempting to speculate that allergic inflammation in the nose predisposes the atopic individual to the development of CRS. Both conditions share the same trend of increasing prevalence (520, 521) and are frequently associated. It has been postulated (522) that swelling of the nasal mucosa in allergic rhinitis at the site of the sinus ostia may compromise ventilation and even obstruct sinus ostia, leading to mucus retention and infection. Furthermore, there has been an increase in the body of opinion that regard the mucosa of the nasal airway as being in a continuum with the paranasal sinuses and hence the term 'rhinosinusitis' was introduced (523). However, critical analysis of the papers linking atopy as a risk factor to CRS reveal that whilst many of the studies suggest a higher prevalence of allergy in patients presenting with symptoms consistent with rhinosinusitis than would be expected in the general population, there may well have been a significant selection process, because the doctors involved often had an interest in allergy (524-528).
A number of studies report that markers of atopy are more prevalent in populations with CRS. Benninger reported that 54% of outpatients with CRS had positive skin prick tests (529). Among CRS patients undergoing sinus surgery, the prevalence of positive skin prick tests ranges from 50% to 84%, of which the majority (60%) have multiple sensitivities (64, 530, 531). However, the role of allergy in CRS is questioned by other epidemiologic studies showing no increase in the incidence of infectious CRS during the pollen season in pollen-sensitized patients (532). Taken together, epidemiologic data show an increased prevalence of allergic rhinitis in patients with CRS, but the role of allergy in CRS remains unclear. Notwithstanding the lack of hard epidemiologic evidence for a clear causal relationship between allergy and CRS, it is clear that failure to address allergy as a contributing factor to CRS diminishes the probability of success of a surgical intervention (533). Among allergy patients undergoing immunotherapy, those who felt most helped by immunotherapy were the subjects with a history of recurrent rhinosinusitis, and about half of the patients, who had had sinus surgery before, believed that the surgery alone was not sufficient to completely resolve the recurrent episodes of infection (533).
Between 0.5 to 4.5% of subjects with allergic rhinitis have NP (505, 534, 535), which compares with the normal population (536). Kern found NP in 25.6% of patients with allergy compared to 3.9% in a control population (536). On the other hand, the prevalence of allergy in patients with NP has been reported as varying from 10% (537), to 54% (538) and 64% (539). Contrary to reports that have implicated atopy as being more prevalent in patients with NP, others have failed to show this (513, 535, 539-541). Recently, Bachert at al. (542) found an association between levels of both total and specific IgE and eosinophilic infiltration in NP. These findings were unrelated to skin prick test results.
Although intradermal test to food allergens are known to be unreliable, positive intradermal tests to food allergens have been reported in 70 % (543) and 81% (544) of NP patients compared to respectively 34% and 11% of controls. Based on questionnaires, food allergy was reported by 22% (496) and 31% (508) of patients with NP, which was significantly higher than in non-NP controls (496). Pang et al. found a higher prevalence of positive intradermal food tests (81%) in patients with NP compared to 11% in a small control group (545). Further research is needed to investigate a possible role for food allergy in the initiation and perpetuation of NP.
Considerable overlap between asthma and nasal comorbidities confirm a close relationship between nasal disease and asthma.
4.1.4.1. Ciliary impairment
As may be concluded from the section on anatomy and pathophysiology, ciliary function plays an important role in the clearance of the sinuses and the prevention of chronic inflammation. Secondary ciliary dyskinesia is found in patients with CRS, and is probably reversible, although restoration takes some time (516). As expected in patients with Kartagener's syndrome and primary ciliary dyskinesia, CRS is a common problem and these patients usually have a long history of respiratory infections.
In patients with cystic fibrosis (CF), the inability of the cilia to transport the viscous mucus causes ciliary malfunction and consequently CRS. NPs are present in about 40% of patients with CF (517). These polyps are generally more neutrophilic than eosinophilic in nature.
4.1.4.2. Allergy
Review articles on CRS have suggested that atopy predisposes to its development (518 , 519). It is tempting to speculate that allergic inflammation in the nose predisposes the atopic individual to the development of CRS. Both conditions share the same trend of increasing prevalence (520, 521) and are frequently associated. It has been postulated (522) that swelling of the nasal mucosa in allergic rhinitis at the site of the sinus ostia may compromise ventilation and even obstruct sinus ostia, leading to mucus retention and infection. Furthermore, there has been an increase in the body of opinion that regard the mucosa of the nasal airway as being in a continuum with the paranasal sinuses and hence the term 'rhinosinusitis' was introduced (523). However, critical analysis of the papers linking atopy as a risk factor to CRS reveal that whilst many of the studies suggest a higher prevalence of allergy in patients presenting with symptoms consistent with rhinosinusitis than would be expected in the general population, there may well have been a significant selection process, because the doctors involved often had an interest in allergy (524-528).
A number of studies report that markers of atopy are more prevalent in populations with CRS. Benninger reported that 54% of outpatients with CRS had positive skin prick tests (529). Among CRS patients undergoing sinus surgery, the prevalence of positive skin prick tests ranges from 50% to 84%, of which the majority (60%) have multiple sensitivities (64, 530, 531). However, the role of allergy in CRS is questioned by other epidemiologic studies showing no increase in the incidence of infectious CRS during the pollen season in pollen-sensitized patients (532). Taken together, epidemiologic data show an increased prevalence of allergic rhinitis in patients with CRS, but the role of allergy in CRS remains unclear. Notwithstanding the lack of hard epidemiologic evidence for a clear causal relationship between allergy and CRS, it is clear that failure to address allergy as a contributing factor to CRS diminishes the probability of success of a surgical intervention (533). Among allergy patients undergoing immunotherapy, those who felt most helped by immunotherapy were the subjects with a history of recurrent rhinosinusitis, and about half of the patients, who had had sinus surgery before, believed that the surgery alone was not sufficient to completely resolve the recurrent episodes of infection (533).
Between 0.5 to 4.5% of subjects with allergic rhinitis have NP (505, 534, 535), which compares with the normal population (536). Kern found NP in 25.6% of patients with allergy compared to 3.9% in a control population (536). On the other hand, the prevalence of allergy in patients with NP has been reported as varying from 10% (537), to 54% (538) and 64% (539). Contrary to reports that have implicated atopy as being more prevalent in patients with NP, others have failed to show this (513, 535, 539-541). Recently, Bachert at al. (542) found an association between levels of both total and specific IgE and eosinophilic infiltration in NP. These findings were unrelated to skin prick test results.
Although intradermal test to food allergens are known to be unreliable, positive intradermal tests to food allergens have been reported in 70 % (543) and 81% (544) of NP patients compared to respectively 34% and 11% of controls. Based on questionnaires, food allergy was reported by 22% (496) and 31% (508) of patients with NP, which was significantly higher than in non-NP controls (496). Pang et al. found a higher prevalence of positive intradermal food tests (81%) in patients with NP compared to 11% in a small control group (545). Further research is needed to investigate a possible role for food allergy in the initiation and perpetuation of NP.
Considerable overlap between asthma and nasal comorbidities confirm a close relationship between nasal disease and asthma.
4.1.4.3. Asthma
CRSwNP and asthma are also frequently associated in the same patients, but their inter-relationship is poorly understood (318). Studies on radiographic abnormalities of the sinuses in asthmatic patients have shown a high prevalence of abnormal sinus mucosa (545, 546). All patients with steroid-dependant asthma had abnormal mucosal changes on CT compared to 88% with mild to moderate asthma (547). GA2LEN studied over 52,000 adults aged 18-75 years and living in 19 centres in 12 countries and concluded that there was a strong association of asthma with CRS. The association with asthma was stronger in those reporting both CRS and allergic rhinitis (13).
Wheezing and respiratory discomfort are present in 31% and 42% of patients with CRSwNP, and asthma is reported by 26% of patients with CRSwNP, compared to 6% of controls (496, 548). Alternatively, 7% of asthmatic patients have NP (505), with a prevalence of 13% in non-atopic asthma and 5% in atopic asthma (513). NP take between 9 and 13 years to develop, but only two years in aspirin-induced asthma (515). Ten percent develop both polyps and asthma simultaneously and the remainder develop polyps first and asthma later (506). Women that have nasal polyps are 1.6 times more likely to be asthmatic and 2.7 times to have allergic rhinitis (509). Asthmatic patients with CRSwNP have more nasal symptoms. Alobid et al. (549) showed that patients with CRSwNP have an impaired sense of smell, that asthma -particularly persistent asthma- has a further impact on sense of smell, and that loss of smell may be used as a clinical tool to identify the severity of both NP and asthma.
4.1.4.4. Aspirin sensitivity
In patients with aspirin sensitivity, 36-96% have CRSwNP.
In patients with aspirin sensitivity 36-96% have CRSwNP (513, 534, 551-555) and up to 96% have radiographic changes affecting their paranasal sinuses (556). Patients with aspirin sensitivity, asthma and NP are usually non-atopic and the prevalence increases over the age of 40 years. The children of patients with asthma, NP, and aspirin sensitivity had NP and rhinosinusitis more often than the children of controls (557). Concerning hereditary factors, HLA A1/B8 has been reported as having a higher incidence in patients with asthma and aspirin sensitivity (558) although Klossek et al. (496) found no difference between gender in 10,033 patients. Zhang et al. (559) found that IgE antibodies to enterotoxins can be found in the majority of NP patients who are aspirin sensitive.
4.1.4.5. Immunocompromised state
Among conditions associated with dysfunction of the immune system, congenital immunodeficiencies manifest themselves with symptoms early in life. However, dysfunction of the immune system may occur later in life and present with CRS. In a retrospective review of refractory sinusitis patients, Chee et al. (560) found an unexpectedly high incidence of immune dysfunction. Of the 60 patients with in vitro T-lymphocyte function testing, 55% showed abnormal proliferation in response to recall antigens. Low immunoglobulin (Ig), IgA and IgM titres were found in 18%, 17%, and 5%, respectively, of patients with refractory sinusitis. Common variable immunodeficiency was diagnosed in 10% and selective IgA deficiency in 6% of patients. Therefore, immunological testing should be an integral part of the diagnostic pathway of patients with CRS. In a cross-sectional study to assess the overall prevalence of otolaryngologic diseases in patients with HIV infection, Porter et al. (561) reported that rhinosinusitis was present in more than half of the HIV-positive population, ranking this condition one of the most prevalent diseases in HIV-positive individuals. However, the relevance of these data is questioned as there was no difference in sinonasal symptom severity between HIV-positive and AIDS patients nor was there a correlation between CD4+ cell counts and symptom severity. In a more detailed study, Garcia-Rodrigues et al. (562) reported a lower incidence of CRS (34%), but with a good correlation between low CD4+ cell count and the probability of CRS. It should also be mentioned here that atypical organisms like Aspergillus spp, Pseudomonas aeruginosa and microsporidia are often isolated from affected sinuses and that neoplasms such as non-Hodgkin lymphoma and Kaposi's sarcoma, may account for sinonasal problems in patients with AIDS (563).
4.1.4.6. Genetic factors. (See also section 4.5)
Although CRSsNP has been observed in family members, no genetic abnormality has been identified linked to CRS. However, the role of genetic factors in CRS has been implicated in patients with cystic fibrosis and primary ciliary dyskinesia (564) and there is some evidence in CRSwNP.
4.1.4.7. Pregnancy and endocrine state
During pregnancy, nasal congestion occurs in approximately one-fifth of women (565). The pathogenesis of this disorder remains unexplained, but there have been a number of proposed theories. Besides direct hormonal effects of oestrogen, progesterone and placental growth hormone on the nasal mucosa, indirect hormonal effects such as vascular changes may be involved. Whether pregnancy rhinitis predisposes to the development of rhinosinusitis, is not clear. In a small prospective study, Sobol et al. (566) report that 61% of pregnant women had nasal congestion during the first trimester, whereas only 3% had sinusitis. In this study, a similar percentage of non-pregnant women in the control group developed sinusitis during the period of the study. Also in an earlier report, the incidence of sinusitis in pregnancy was shown to be quite low, i.e. 1.5% (567). In addition, thyroid dysfunction has been implicated in CRS, but there is only limited data on the prevalence of CRS in patients with hypothyroidism.
4.1.4.8. Local host factors
There is no evidence for a causal correlation between nasal anatomic variations in general and the incidence of CRS.
Certain anatomic variations such as concha bullosa, nasal septal deviation and a displaced uncinate process, have been suggested as potential risk factors for developing CRS (568). However, some of the studies that have made this assertion have equated mucosal thickening on CT with CRS (569) when it has been shown that incidental mucosal thickening occurs in approximately a third of an asymptomatic population (570).Bolger et al. (571) and Nouraei et al. (572) found no correlation between CRS and bony anatomic variations in the nose. Holbrook et al (573) also found no correlation between sinus opacification, anatomical variations and symptom scores. Nonetheless, one should mention here that no study has so far investigated whether a particular anatomic variation can impair drainage of the ostiomeatal complex per se. Whilst some authors have postulated that anatomical variations of the paranasal sinuses can contribute to ostial obstruction (574) there are several studies that show the prevalence of anatomical variations is no more common in patients with CRSs or wNP than in a control population (570, 575, 576).
One area where conjecture remains is the effect of a deviated septum. There are a number of studies that show no correlation between septal deviation and the prevalence of CRS (498, 577). Whilst there is no recognised method of objectively defining the extent of a deviated septum, some studies have found a deviation of more than 3mm from the midline to be more prevalent in rhinosinusitis (578, 579) whilst others have not (575, 577, 580). In spite of the observation that sinonasal complaints often resolve after surgery, this does not necessarily imply that anatomic variation is aetiologically involved. CRS of dental origin should not be overlooked when considering the aetiology of CRS. Obtaining accurate epidemiologic data on the incidence of CRS of dental origin is not possible as the literature is limited to anecdotal reports though there is some evidence that odontogenic sinusitis may be increasing (581). Taken together, there is no evidence for a causal correlation between nasal anatomic variations in general and the incidence of CRS.
4.1.4.9. Biofilms (See also section 4.2)
Many pathogenic bacteria colonize the surface of the NPs forming biofilms. They are not a primary etiologic agent in NP, but a contributor significantly adding more inflammation. Clinically, cases of NP with presence of biofilms are correlated with severe forms of the disease and worse postoperative outcome (550, 582).
Methicillin-resistant Staphylococcus aureus (MRSA) does not appear to pose a significant risk of morbidity in our patient population. However, ongoing concern regarding the increasing prevalence of S. aureus and antimicrobial resistance in chronic sinonasal disease highlights the importance of using culturedirected antimicrobial therapy with the goal of minimizing future resistance patterns (583) Bhattacharyya and Kepnes (584) analyzed 701 bacterial isolates among 392 culture samples from patients with CRS. They concluded that antibiotic resistance seems to be emerging for erythromycin at a rate higher than for other antibiotics like methicillin, clindamycin, gentamicin, tetracycline, sulphamethoxazole, and levofloxacin. Although not increasing in prevalence, MRSA maintains a significant presence in CRS with associated increased levels of antibiotic resistance. Bachert et al. (585) investigated 70 patients and demonstrated that mucosal inflammation in nasal polyps orchestrated by Th2 cytokines and amplified by S. aureus enterotoxins is characterized by an increased eosinophilic inflammation and formation of IgE antibodies.
4.1.4.10. Environmental factors (See also section 4.2.)
Cigarette smoking was associated with a higher prevalence of CRS in Canada (11) and exposure to secondhand smoke is common and significantly independently associated with CRS (561), whereas this observation was not confirmed in a nationwide survey in Korea (489). GA(2)LEN study demonstrated that smoking was associated with having CRS in all parts of Europe (GALEN study) (492). Recently, other lifestyle-related factors are undoubtedly involved in the chronic inflammatory processes of CRSsNP. For instance, low income was associated with a higher prevalence of CRSsNP (11). In spite of in vitro data on the toxicity of pollutants on respiratory epithelium, there exists no convincing evidence for the aetiologic role of pollutants and toxins such as ozone in CRSsNP. Koh et al. (562) investigated the relationship between CRS and occupation and concluded that there were significantly increased prevalence ratios of CRS in plant and machinery operators and assemblers, elementary occupations, crafts and related trade workers, and the unemployed.
The role of environmental factors in the development of CRSwNP is unclear. No difference in the prevalence of CRSwNP has been found related to the patient's habitat or pollution at work (508). One study found that a significantly smaller proportion of the population with polyps were smokers compared to an unselected population (15% vs. 35%) (508), whereas this was not confirmed by others (496). One study reports on the association between the use of a woodstove as a primary source of heating and the development of NP (586).
4.1.4.11. Iatrogenic factors
Among risk factors of CRS, iatrogenic factors should not be forgotten as they may be responsible for the failure of sinus surgery. The increasing number of sinus mucocoeles seems to correlate with the increase in endoscopic sinus surgery procedures. Among a group of 42 patients with mucocoeles, 11 had prior surgery within 2 years prior to presentation (587). Another reason for failure after surgery can be the recirculation of mucus out of the natural maxillary ostium and back through a separate surgically created antrostomy resulting in an increased risk of persistent sinus infection (588).
4.1.4.12. Helicobacter pylori and laryngopharyngeal reflux
H. pylori DNA has been detected in between 11% (589) 33% of sinus samples from patients with CRSsNP but not from controls (590). Flook and Kumar (105) reviewed nineteen papers describing varying studies on CRS and acid reflux. There is not enough evidence to consider anti-reflux therapy for adult refractory CRS and there is no evidence that acid reflux is a significant causal factor in CRSsNP.
4.1.4.13. "Osteitis"
This is considered fully in Section 5b but the study by Telmesani and al-Shawarby (591) is noteworthy. They studied 50 patients undergoing FESS for the first time and 32 patients undergoing revision surgery. Histopathological examination was performed for specimens taken from the bony septa of the ethmoid with the overlying mucosa. Bony changes were seen in only 30% of primary NP compared to 87.5% in recurrent cases.
CRSwNP and asthma are also frequently associated in the same patients, but their inter-relationship is poorly understood (318). Studies on radiographic abnormalities of the sinuses in asthmatic patients have shown a high prevalence of abnormal sinus mucosa (545, 546). All patients with steroid-dependant asthma had abnormal mucosal changes on CT compared to 88% with mild to moderate asthma (547). GA2LEN studied over 52,000 adults aged 18-75 years and living in 19 centres in 12 countries and concluded that there was a strong association of asthma with CRS. The association with asthma was stronger in those reporting both CRS and allergic rhinitis (13).
Wheezing and respiratory discomfort are present in 31% and 42% of patients with CRSwNP, and asthma is reported by 26% of patients with CRSwNP, compared to 6% of controls (496, 548). Alternatively, 7% of asthmatic patients have NP (505), with a prevalence of 13% in non-atopic asthma and 5% in atopic asthma (513). NP take between 9 and 13 years to develop, but only two years in aspirin-induced asthma (515). Ten percent develop both polyps and asthma simultaneously and the remainder develop polyps first and asthma later (506). Women that have nasal polyps are 1.6 times more likely to be asthmatic and 2.7 times to have allergic rhinitis (509). Asthmatic patients with CRSwNP have more nasal symptoms. Alobid et al. (549) showed that patients with CRSwNP have an impaired sense of smell, that asthma -particularly persistent asthma- has a further impact on sense of smell, and that loss of smell may be used as a clinical tool to identify the severity of both NP and asthma.
4.1.4.4. Aspirin sensitivity
In patients with aspirin sensitivity, 36-96% have CRSwNP.
In patients with aspirin sensitivity 36-96% have CRSwNP (513, 534, 551-555) and up to 96% have radiographic changes affecting their paranasal sinuses (556). Patients with aspirin sensitivity, asthma and NP are usually non-atopic and the prevalence increases over the age of 40 years. The children of patients with asthma, NP, and aspirin sensitivity had NP and rhinosinusitis more often than the children of controls (557). Concerning hereditary factors, HLA A1/B8 has been reported as having a higher incidence in patients with asthma and aspirin sensitivity (558) although Klossek et al. (496) found no difference between gender in 10,033 patients. Zhang et al. (559) found that IgE antibodies to enterotoxins can be found in the majority of NP patients who are aspirin sensitive.
4.1.4.5. Immunocompromised state
Among conditions associated with dysfunction of the immune system, congenital immunodeficiencies manifest themselves with symptoms early in life. However, dysfunction of the immune system may occur later in life and present with CRS. In a retrospective review of refractory sinusitis patients, Chee et al. (560) found an unexpectedly high incidence of immune dysfunction. Of the 60 patients with in vitro T-lymphocyte function testing, 55% showed abnormal proliferation in response to recall antigens. Low immunoglobulin (Ig), IgA and IgM titres were found in 18%, 17%, and 5%, respectively, of patients with refractory sinusitis. Common variable immunodeficiency was diagnosed in 10% and selective IgA deficiency in 6% of patients. Therefore, immunological testing should be an integral part of the diagnostic pathway of patients with CRS. In a cross-sectional study to assess the overall prevalence of otolaryngologic diseases in patients with HIV infection, Porter et al. (561) reported that rhinosinusitis was present in more than half of the HIV-positive population, ranking this condition one of the most prevalent diseases in HIV-positive individuals. However, the relevance of these data is questioned as there was no difference in sinonasal symptom severity between HIV-positive and AIDS patients nor was there a correlation between CD4+ cell counts and symptom severity. In a more detailed study, Garcia-Rodrigues et al. (562) reported a lower incidence of CRS (34%), but with a good correlation between low CD4+ cell count and the probability of CRS. It should also be mentioned here that atypical organisms like Aspergillus spp, Pseudomonas aeruginosa and microsporidia are often isolated from affected sinuses and that neoplasms such as non-Hodgkin lymphoma and Kaposi's sarcoma, may account for sinonasal problems in patients with AIDS (563).
4.1.4.6. Genetic factors. (See also section 4.5)
Although CRSsNP has been observed in family members, no genetic abnormality has been identified linked to CRS. However, the role of genetic factors in CRS has been implicated in patients with cystic fibrosis and primary ciliary dyskinesia (564) and there is some evidence in CRSwNP.
4.1.4.7. Pregnancy and endocrine state
During pregnancy, nasal congestion occurs in approximately one-fifth of women (565). The pathogenesis of this disorder remains unexplained, but there have been a number of proposed theories. Besides direct hormonal effects of oestrogen, progesterone and placental growth hormone on the nasal mucosa, indirect hormonal effects such as vascular changes may be involved. Whether pregnancy rhinitis predisposes to the development of rhinosinusitis, is not clear. In a small prospective study, Sobol et al. (566) report that 61% of pregnant women had nasal congestion during the first trimester, whereas only 3% had sinusitis. In this study, a similar percentage of non-pregnant women in the control group developed sinusitis during the period of the study. Also in an earlier report, the incidence of sinusitis in pregnancy was shown to be quite low, i.e. 1.5% (567). In addition, thyroid dysfunction has been implicated in CRS, but there is only limited data on the prevalence of CRS in patients with hypothyroidism.
4.1.4.8. Local host factors
There is no evidence for a causal correlation between nasal anatomic variations in general and the incidence of CRS.
Certain anatomic variations such as concha bullosa, nasal septal deviation and a displaced uncinate process, have been suggested as potential risk factors for developing CRS (568). However, some of the studies that have made this assertion have equated mucosal thickening on CT with CRS (569) when it has been shown that incidental mucosal thickening occurs in approximately a third of an asymptomatic population (570).Bolger et al. (571) and Nouraei et al. (572) found no correlation between CRS and bony anatomic variations in the nose. Holbrook et al (573) also found no correlation between sinus opacification, anatomical variations and symptom scores. Nonetheless, one should mention here that no study has so far investigated whether a particular anatomic variation can impair drainage of the ostiomeatal complex per se. Whilst some authors have postulated that anatomical variations of the paranasal sinuses can contribute to ostial obstruction (574) there are several studies that show the prevalence of anatomical variations is no more common in patients with CRSs or wNP than in a control population (570, 575, 576).
One area where conjecture remains is the effect of a deviated septum. There are a number of studies that show no correlation between septal deviation and the prevalence of CRS (498, 577). Whilst there is no recognised method of objectively defining the extent of a deviated septum, some studies have found a deviation of more than 3mm from the midline to be more prevalent in rhinosinusitis (578, 579) whilst others have not (575, 577, 580). In spite of the observation that sinonasal complaints often resolve after surgery, this does not necessarily imply that anatomic variation is aetiologically involved. CRS of dental origin should not be overlooked when considering the aetiology of CRS. Obtaining accurate epidemiologic data on the incidence of CRS of dental origin is not possible as the literature is limited to anecdotal reports though there is some evidence that odontogenic sinusitis may be increasing (581). Taken together, there is no evidence for a causal correlation between nasal anatomic variations in general and the incidence of CRS.
4.1.4.9. Biofilms (See also section 4.2)
Many pathogenic bacteria colonize the surface of the NPs forming biofilms. They are not a primary etiologic agent in NP, but a contributor significantly adding more inflammation. Clinically, cases of NP with presence of biofilms are correlated with severe forms of the disease and worse postoperative outcome (550, 582).
Methicillin-resistant Staphylococcus aureus (MRSA) does not appear to pose a significant risk of morbidity in our patient population. However, ongoing concern regarding the increasing prevalence of S. aureus and antimicrobial resistance in chronic sinonasal disease highlights the importance of using culturedirected antimicrobial therapy with the goal of minimizing future resistance patterns (583) Bhattacharyya and Kepnes (584) analyzed 701 bacterial isolates among 392 culture samples from patients with CRS. They concluded that antibiotic resistance seems to be emerging for erythromycin at a rate higher than for other antibiotics like methicillin, clindamycin, gentamicin, tetracycline, sulphamethoxazole, and levofloxacin. Although not increasing in prevalence, MRSA maintains a significant presence in CRS with associated increased levels of antibiotic resistance. Bachert et al. (585) investigated 70 patients and demonstrated that mucosal inflammation in nasal polyps orchestrated by Th2 cytokines and amplified by S. aureus enterotoxins is characterized by an increased eosinophilic inflammation and formation of IgE antibodies.
4.1.4.10. Environmental factors (See also section 4.2.)
Cigarette smoking was associated with a higher prevalence of CRS in Canada (11) and exposure to secondhand smoke is common and significantly independently associated with CRS (561), whereas this observation was not confirmed in a nationwide survey in Korea (489). GA(2)LEN study demonstrated that smoking was associated with having CRS in all parts of Europe (GALEN study) (492). Recently, other lifestyle-related factors are undoubtedly involved in the chronic inflammatory processes of CRSsNP. For instance, low income was associated with a higher prevalence of CRSsNP (11). In spite of in vitro data on the toxicity of pollutants on respiratory epithelium, there exists no convincing evidence for the aetiologic role of pollutants and toxins such as ozone in CRSsNP. Koh et al. (562) investigated the relationship between CRS and occupation and concluded that there were significantly increased prevalence ratios of CRS in plant and machinery operators and assemblers, elementary occupations, crafts and related trade workers, and the unemployed.
The role of environmental factors in the development of CRSwNP is unclear. No difference in the prevalence of CRSwNP has been found related to the patient's habitat or pollution at work (508). One study found that a significantly smaller proportion of the population with polyps were smokers compared to an unselected population (15% vs. 35%) (508), whereas this was not confirmed by others (496). One study reports on the association between the use of a woodstove as a primary source of heating and the development of NP (586).
4.1.4.11. Iatrogenic factors
Among risk factors of CRS, iatrogenic factors should not be forgotten as they may be responsible for the failure of sinus surgery. The increasing number of sinus mucocoeles seems to correlate with the increase in endoscopic sinus surgery procedures. Among a group of 42 patients with mucocoeles, 11 had prior surgery within 2 years prior to presentation (587). Another reason for failure after surgery can be the recirculation of mucus out of the natural maxillary ostium and back through a separate surgically created antrostomy resulting in an increased risk of persistent sinus infection (588).
4.1.4.12. Helicobacter pylori and laryngopharyngeal reflux
H. pylori DNA has been detected in between 11% (589) 33% of sinus samples from patients with CRSsNP but not from controls (590). Flook and Kumar (105) reviewed nineteen papers describing varying studies on CRS and acid reflux. There is not enough evidence to consider anti-reflux therapy for adult refractory CRS and there is no evidence that acid reflux is a significant causal factor in CRSsNP.
4.1.4.13. "Osteitis"
This is considered fully in Section 5b but the study by Telmesani and al-Shawarby (591) is noteworthy. They studied 50 patients undergoing FESS for the first time and 32 patients undergoing revision surgery. Histopathological examination was performed for specimens taken from the bony septa of the ethmoid with the overlying mucosa. Bony changes were seen in only 30% of primary NP compared to 87.5% in recurrent cases.
4.2. Inflammatory mechanisms in chronic rhinosinusitis with
or without polyps (CRSwNP or CRSsNP)
4.2.1. Summary: Aetiology and Pathogenesis of CRS
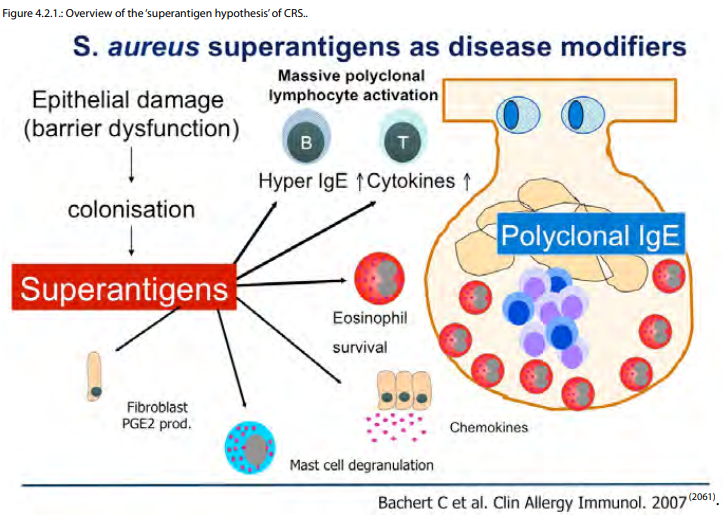
Historically, idiopathic CRS was attributed to either the end stage of an incompletely treated case of acute RS (CRSsNP) or severe atopy (CRSwNP). The limitations of these assessments were clear to many but relatively few hypotheses have been proposed as alternatives. The first attempt to address aetiology and pathogenesis in broad terms was the 'fungal hypothesis', which attributed all CRS to an excessive host response to Alternaria fungi (592, 593). Although most investigators have rejected the basic tenets as originally proposed, fungi are still believed by many to play a role as a disease modifier in at least some forms of CRS. Defects in the eicosanoid pathway, most closely associated with aspirin intolerance, have also been proposed as a potential cause of CRSwNP in general (594, 595). Specifically, increased synthesis of pro-inflammatory leukotrienes and decreased synthesis of anti-inflammatory prostaglandins (PGE2) have been proposed as a mechanism not just for aspirin-sensitive nasal polyps but also aspirin-tolerant CRSwNP. While some theoretical evidence supports this line of thought in CRSwNP, enthusiasm is muted by the limited clinical efficacy of leukotriene pathway inhibitors. The 'staphylococcal superantigen hypothesis' proposed that exotoxins foster nasal polyposis via effects on multiple cell types (542, 596). The net effect is Th2 skewing, Treg inhibition, accentuated eosinophil and mast cell activity and heightened tissue damage and remodeling. It remains unclear why superantigen effects can be demonstrated in only approximately half of CRSwNP patients; hence, staphylococcal superantigens are generally seen by many as disease modifiers, rather than discrete aetiologic agents (594). The 'immune barrier hypothesis' proposed that defects in the co-ordinated mechanical barrier and/or the innate immune response of the sinonasal epithelium manifests as CRS (25). These defects theoretically lead to increased microbial colonization with a panoply of microbial agents, accentuated barrier damage and a compensatory adaptive immune response (597). One potential molecular mechanism for this hypothesis would include local defects in the STAT 3 pathway, which has been identified in some forms of CRS (598). Systemic defects in STAT 3 have been identified in Job's disease, a disorder with some striking similarities to CRSwNP (599). The 'immune barrier hypothesis' does not specifically address the Th subset skewing observed in many CRS subtypes, including the Th2 pattern and B cell infiltrate observed in Western CRSwNP patients. This implies additional, as yet undetermined, mechanisms or defects that foster an inappropriate local, adaptive response in the sinonasal mucosa. Genes that may be involved in Th2 skewing include TSLP, IL-33, IL-25 and genes in the strong B cell response include BAFF, CXCL12 and CXCL13 (600-602). An excessive and/or inappropriate Th2 adaptive response in this setting may further compromise barrier function and diminish innate immunity, thereby creating a self-perpetuating cycle of disease. In the most severe forms of CRSwNP, new evidence supports the generation of local autoantibodies further accentuating tissue damage (23). Lastly, biofilms have been suggested as a potential entity that can cause CRS (603). It can be speculated that a defect in the immune barrier might facilitate formation of biofilms. The mechanism of biofilm formation and worsening of CRS remain unclear but biofilms on the sinus mucosa have been likened to those mediating periodontal disease (604).
Epithelial damage and/or host barrier dysfunction results in colonization with S. aureus. Subsequent secretion of superantigenic toxins has effects on multiple cell types including epithelial cells, lymphocytes, eosinophils, fibroblasts and mast cells. Locally, the net effect is to help the organism evade the host immune response. The primary host effects are a skewing of the inflammatory response in the Th2 direction, generation of local polyclonal IgE, promotion of eosinophil survival and mast cell degranulation and alteration of eicosanoid metabolism. The sum of these local tissue effects is believed to foster polyp formation. The capability of S. aureus to reside within airway epithelial cells likely only augments this process.
CRS can be typically described as a dysfunctional host-environment interaction at the site of interface, which occurs in the nose and paranasal sinuses
The current hypotheses that discuss CRS aetiology and pathogenesis are less in conflict than might appear. Superantigens for example, have been shown to modulate eicosanoid metabolism (605, 606) suggesting a link between two of the proposed theories. Furthermore, the presence of intracellular S. aureus in epithelial cells from CRSwNP but not CRSsNP or controls, suggests defective local immune and/or barrier function (607, 608). One mechanism may be the induction of M2 macrophages, which have diminished phagocytic properties, by enhanced local Th2 immunity induced by superantigens (594, 609, 610). It has been suggested that S. aureus biofilms have the ability to skew the cytokine milieu in the Th2 direction independently of superantigens (603). Lastly, fungi have substantial intrinsic protease activities, which may degrade tight junctions accentuating host barrier compromise (25, 597). The interplay between exogenous agents and host defects conceptually links the theories although the relative importance and validity of various components remains in flux.
Host factors that determine susceptibility to CRS depend, in part, on genetic variation across key pathways governing the immunobiology of the nasal mucosa (25). Cystic fibrosis (CF) is the prototypic case of 'genetic' CRS wherein dysfunction of the CFTR gene triggers defective innate immune and barrier functions (611). In the case of CF, simple Mendelian genetics apply but a wide variation of sinus disease expression is nevertheless observed, despite identical mutations in the CFTR gene (612). Consequently even in CF, the most straightforward case of genetic CRS, multiple genes are involved in an individual patient determining clinical phenotype (613). Early attempts to identify additional genetic loci important in CRS have been undertaken and this is a work in progress (614). Comprehensive genome wide association studies (GWAS) studies have yet to been performed in CRS, but multiple studies have been done in related chronic inflammatory disorders including asthma (615). In terms of aetiology and pathogenesis, these studies as well as others, suggest the involvement of not only multiple genetic loci but also the importance of environmentally-determined epigenetic changes (616-619). Hence, host susceptibility to complex diseases such as CRS likely reflects the combined effects of variation in not only the DNA base sequence but also the DNA methylation and histone modification patterns caused by past environmental exposures. Ongoing environmental stresses confront the susceptible host, which may lead to development of the chronically inflamed state known as CRS.
4.2.1. Summary: Aetiology and Pathogenesis of CRS
Historically, idiopathic CRS was attributed to either the end stage of an incompletely treated case of acute RS (CRSsNP) or severe atopy (CRSwNP). The limitations of these assessments were clear to many but relatively few hypotheses have been proposed as alternatives. The first attempt to address aetiology and pathogenesis in broad terms was the 'fungal hypothesis', which attributed all CRS to an excessive host response to Alternaria fungi (592, 593). Although most investigators have rejected the basic tenets as originally proposed, fungi are still believed by many to play a role as a disease modifier in at least some forms of CRS. Defects in the eicosanoid pathway, most closely associated with aspirin intolerance, have also been proposed as a potential cause of CRSwNP in general (594, 595). Specifically, increased synthesis of pro-inflammatory leukotrienes and decreased synthesis of anti-inflammatory prostaglandins (PGE2) have been proposed as a mechanism not just for aspirin-sensitive nasal polyps but also aspirin-tolerant CRSwNP. While some theoretical evidence supports this line of thought in CRSwNP, enthusiasm is muted by the limited clinical efficacy of leukotriene pathway inhibitors. The 'staphylococcal superantigen hypothesis' proposed that exotoxins foster nasal polyposis via effects on multiple cell types (542, 596). The net effect is Th2 skewing, Treg inhibition, accentuated eosinophil and mast cell activity and heightened tissue damage and remodeling. It remains unclear why superantigen effects can be demonstrated in only approximately half of CRSwNP patients; hence, staphylococcal superantigens are generally seen by many as disease modifiers, rather than discrete aetiologic agents (594). The 'immune barrier hypothesis' proposed that defects in the co-ordinated mechanical barrier and/or the innate immune response of the sinonasal epithelium manifests as CRS (25). These defects theoretically lead to increased microbial colonization with a panoply of microbial agents, accentuated barrier damage and a compensatory adaptive immune response (597). One potential molecular mechanism for this hypothesis would include local defects in the STAT 3 pathway, which has been identified in some forms of CRS (598). Systemic defects in STAT 3 have been identified in Job's disease, a disorder with some striking similarities to CRSwNP (599). The 'immune barrier hypothesis' does not specifically address the Th subset skewing observed in many CRS subtypes, including the Th2 pattern and B cell infiltrate observed in Western CRSwNP patients. This implies additional, as yet undetermined, mechanisms or defects that foster an inappropriate local, adaptive response in the sinonasal mucosa. Genes that may be involved in Th2 skewing include TSLP, IL-33, IL-25 and genes in the strong B cell response include BAFF, CXCL12 and CXCL13 (600-602). An excessive and/or inappropriate Th2 adaptive response in this setting may further compromise barrier function and diminish innate immunity, thereby creating a self-perpetuating cycle of disease. In the most severe forms of CRSwNP, new evidence supports the generation of local autoantibodies further accentuating tissue damage (23). Lastly, biofilms have been suggested as a potential entity that can cause CRS (603). It can be speculated that a defect in the immune barrier might facilitate formation of biofilms. The mechanism of biofilm formation and worsening of CRS remain unclear but biofilms on the sinus mucosa have been likened to those mediating periodontal disease (604).
Epithelial damage and/or host barrier dysfunction results in colonization with S. aureus. Subsequent secretion of superantigenic toxins has effects on multiple cell types including epithelial cells, lymphocytes, eosinophils, fibroblasts and mast cells. Locally, the net effect is to help the organism evade the host immune response. The primary host effects are a skewing of the inflammatory response in the Th2 direction, generation of local polyclonal IgE, promotion of eosinophil survival and mast cell degranulation and alteration of eicosanoid metabolism. The sum of these local tissue effects is believed to foster polyp formation. The capability of S. aureus to reside within airway epithelial cells likely only augments this process.
CRS can be typically described as a dysfunctional host-environment interaction at the site of interface, which occurs in the nose and paranasal sinuses
The current hypotheses that discuss CRS aetiology and pathogenesis are less in conflict than might appear. Superantigens for example, have been shown to modulate eicosanoid metabolism (605, 606) suggesting a link between two of the proposed theories. Furthermore, the presence of intracellular S. aureus in epithelial cells from CRSwNP but not CRSsNP or controls, suggests defective local immune and/or barrier function (607, 608). One mechanism may be the induction of M2 macrophages, which have diminished phagocytic properties, by enhanced local Th2 immunity induced by superantigens (594, 609, 610). It has been suggested that S. aureus biofilms have the ability to skew the cytokine milieu in the Th2 direction independently of superantigens (603). Lastly, fungi have substantial intrinsic protease activities, which may degrade tight junctions accentuating host barrier compromise (25, 597). The interplay between exogenous agents and host defects conceptually links the theories although the relative importance and validity of various components remains in flux.
Host factors that determine susceptibility to CRS depend, in part, on genetic variation across key pathways governing the immunobiology of the nasal mucosa (25). Cystic fibrosis (CF) is the prototypic case of 'genetic' CRS wherein dysfunction of the CFTR gene triggers defective innate immune and barrier functions (611). In the case of CF, simple Mendelian genetics apply but a wide variation of sinus disease expression is nevertheless observed, despite identical mutations in the CFTR gene (612). Consequently even in CF, the most straightforward case of genetic CRS, multiple genes are involved in an individual patient determining clinical phenotype (613). Early attempts to identify additional genetic loci important in CRS have been undertaken and this is a work in progress (614). Comprehensive genome wide association studies (GWAS) studies have yet to been performed in CRS, but multiple studies have been done in related chronic inflammatory disorders including asthma (615). In terms of aetiology and pathogenesis, these studies as well as others, suggest the involvement of not only multiple genetic loci but also the importance of environmentally-determined epigenetic changes (616-619). Hence, host susceptibility to complex diseases such as CRS likely reflects the combined effects of variation in not only the DNA base sequence but also the DNA methylation and histone modification patterns caused by past environmental exposures. Ongoing environmental stresses confront the susceptible host, which may lead to development of the chronically inflamed state known as CRS.
The model of CRS, in which interplay between multiple host
factors and environmental stressors takes centre-stage, makes
the observed variability in inflammatory tissue infiltrates and
clinical phenotype readily explicable. At the time of the last
EPOS review, CRS was divided into CRSsNP, a Th1 disorder, and
CRSwNP, a Th2 disorder (620). More recent studies have
demonstrated that this paradigm does not apply worldwide, in
particular for CRSwNP, as some Asian polyps exhibit Th1, Th17
and KCN cytokine profiles (621).A new hypothesis has been
proposed suggesting that CRSsNP is characterized by fibrosis,
high levels of TGF-β and increased Treg activity while CRSwNP
exhibits oedema, low TGF-β levels and low Treg activity (594,
622). Further studies will be necessary to test the validity of
this revised proposal. Nevertheless, racial and cultural
differences across the globe almost assuredly modulate
susceptibility and response patterns of the host. Variations in
the nasal bacterial colonization patterns observed worldwide
(623) indirectly supports this concept and further suggests that
ongoing environmental stressors likely also vary with culture
and geography.
Since the last EPOS document there has been significant progress toward understanding the aetiology and pathogenesis of CRS. CRS is still described as 'multifactorial' and there is no clearly delineated single molecular pathway that explains the journey from injury to tissue change (20). There is however, an emerging consensus that the persistent inflammation that defines CRS results from a dysfunctional host-environment interaction involving various exogenous agents and changes in the sinonasal mucosa. In concert with the definition of CRS as an inflammatory disorder, there has been movement away from pathogen-driven hypotheses. This overall concept is in agreement with the current understanding of the aetiology and pathogenesis of chronic mucosal inflammatory disorders in general, which describe a balance of interactions between the host, commensal flora, potential pathogens and exogenous stresses (624).
Since the last EPOS document there has been significant progress toward understanding the aetiology and pathogenesis of CRS. CRS is still described as 'multifactorial' and there is no clearly delineated single molecular pathway that explains the journey from injury to tissue change (20). There is however, an emerging consensus that the persistent inflammation that defines CRS results from a dysfunctional host-environment interaction involving various exogenous agents and changes in the sinonasal mucosa. In concert with the definition of CRS as an inflammatory disorder, there has been movement away from pathogen-driven hypotheses. This overall concept is in agreement with the current understanding of the aetiology and pathogenesis of chronic mucosal inflammatory disorders in general, which describe a balance of interactions between the host, commensal flora, potential pathogens and exogenous stresses (624).
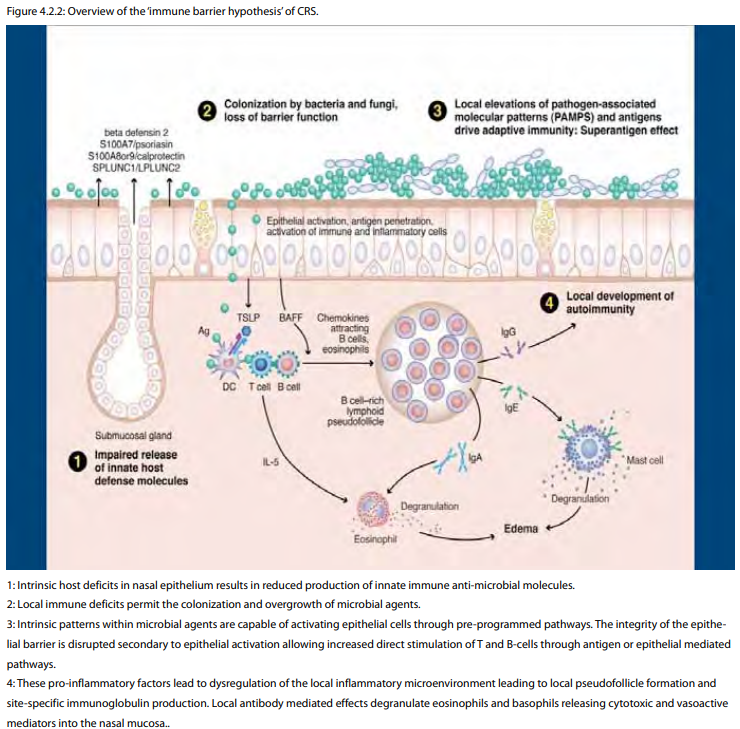
4.2.2. Introduction
Chronic rhinosinusitis (CRS) is a clinical syndrome characterized by persistent symptomatic, inflammation of the mucosa of the nose and paranasal sinuses. The inflammation that defines this disorder occurs at the interface with the external environment, suggesting the still unproven hypothesis that CRS results from an inappropriate or excessive immune response to foreign agents resulting in persistent mucosal inflammation, cellular influx, radiographic changes and clinical disease (25). The widespread adoption of the term 'rhinosinusitis' in preference to 'sinusitis' indirectly supports the perspective that foreign material brought in through the airway, or perhaps from the nasopharynx, acts on the nasal mucosa first, with secondary effects-direct and indirect-on the sinus mucosa (14, 594). In a very small percentage of cases such as dental or iatrogenic sinusitis, this pathway is reversed with processes in the sinus cavity leading to secondary nasal inflammation. CRS may also, in rare cases, develop secondary to inflammatory processes intrinsic to the mucosa in the presumed absence of exogenous stimuli (e.g. Wegener's granulomatosis, sarcoidosis). Lastly, CRS may occur in association with distinct host genetic factors (cystic fibrosis) or systemic immunodeficiency. In the overwhelming majority of CRS cases however, the etiology and pathogenesis remains unclear. This section will focus on idiopathic CRS, with references to other better-defined inflammatory disorders only as they reveal general principles of the immune response of the sinonasal mucosa.
Idiopathic CRS has been typically divided into CRSsNP and CRSwNP based on endoscopic findings. In terms of aetiology and pathogenesis, CRSsNP is more tightly linked to mechanical obstruction of the ostio-meatal complex (OMC) while CRSwNP is generally attributed to a more diffuse mucosal response (625). A minority of investigators still hold that the distinction between the two groups is primarily one of disease-intensity and duration (20, 626, 627). The weight of current research however, would suggest separate, but likely overlapping inflammatory mechanisms and for research purposes this separation facilitates data analysis and determination of molecular pathways of disease (628). Most investigators, and most lines of research however, assume that the inflammation seen in idiopathic CRS results primarily from a dysfunctional host-environment interaction (25). Identification of the exogenous agents, which drive the secondary inflammatory mechanisms, has been a major research focus for many years. This section will provide an overview of currently proposed environmental inflammatory triggers followed by a review of the literature concerning the host mucosal response in CRS, separating out specific agents and mechanisms based on disease phenotype to the extent currently possible.
Chronic rhinosinusitis (CRS) is a clinical syndrome characterized by persistent symptomatic, inflammation of the mucosa of the nose and paranasal sinuses. The inflammation that defines this disorder occurs at the interface with the external environment, suggesting the still unproven hypothesis that CRS results from an inappropriate or excessive immune response to foreign agents resulting in persistent mucosal inflammation, cellular influx, radiographic changes and clinical disease (25). The widespread adoption of the term 'rhinosinusitis' in preference to 'sinusitis' indirectly supports the perspective that foreign material brought in through the airway, or perhaps from the nasopharynx, acts on the nasal mucosa first, with secondary effects-direct and indirect-on the sinus mucosa (14, 594). In a very small percentage of cases such as dental or iatrogenic sinusitis, this pathway is reversed with processes in the sinus cavity leading to secondary nasal inflammation. CRS may also, in rare cases, develop secondary to inflammatory processes intrinsic to the mucosa in the presumed absence of exogenous stimuli (e.g. Wegener's granulomatosis, sarcoidosis). Lastly, CRS may occur in association with distinct host genetic factors (cystic fibrosis) or systemic immunodeficiency. In the overwhelming majority of CRS cases however, the etiology and pathogenesis remains unclear. This section will focus on idiopathic CRS, with references to other better-defined inflammatory disorders only as they reveal general principles of the immune response of the sinonasal mucosa.
Idiopathic CRS has been typically divided into CRSsNP and CRSwNP based on endoscopic findings. In terms of aetiology and pathogenesis, CRSsNP is more tightly linked to mechanical obstruction of the ostio-meatal complex (OMC) while CRSwNP is generally attributed to a more diffuse mucosal response (625). A minority of investigators still hold that the distinction between the two groups is primarily one of disease-intensity and duration (20, 626, 627). The weight of current research however, would suggest separate, but likely overlapping inflammatory mechanisms and for research purposes this separation facilitates data analysis and determination of molecular pathways of disease (628). Most investigators, and most lines of research however, assume that the inflammation seen in idiopathic CRS results primarily from a dysfunctional host-environment interaction (25). Identification of the exogenous agents, which drive the secondary inflammatory mechanisms, has been a major research focus for many years. This section will provide an overview of currently proposed environmental inflammatory triggers followed by a review of the literature concerning the host mucosal response in CRS, separating out specific agents and mechanisms based on disease phenotype to the extent currently possible.
4.2.3. Inflammatory triggers
4.2.3.1. Bacteria
Bacteria have an established role in the aetiology of acute rhinosinusitis (ARS) and it has long been speculated that incompletely treated bacterial ARS leads to the development of CRS. While bacteria may well trigger acute infectious exacerbations, the role of bacteria in the initial establishment of CRS remains unclear. This section will provide an overview of evidence for and against bacteria as aetiologic agents in CRS with emphasis on recent data.
The nasal microbiota is complex and multiple methods, with varying degrees of sensitivity and specificity, have been utilized to determine the bacterial density and composition in health and disease (629). Analysis of samples obtained from the vestibule, measured using molecular techniques, demonstrate multiple bacterial species but a preponderance of the staphylococci and corynebacterium (630, 631). An inverse correlation between the two families was observed, suggesting an antagonistic relationship (632). In addition, the presence of S. epidermidis appears to compete with S. aureus (633). The normal microbiota of the middle meatus may, of course, be quite different than the anterior nostril but these principles likely apply. Healthy sinus cavities, studied using conventional techniques only, appear to have substantially less bacterial colonization than the nasal airway (634). Although not yet tested, more sensitive techniques would likely reveal the presence of a significant bacterial load in the sinuses, given the documented colonization of the lower airway even in healthy individuals (630). Colonizing commensal bacteria in the nose and possibly the sinuses may be important not only in interfering with the growth of pathogens, but also modulating the host immune response (635). This latter effect has been studied in the mouse airway. Animals reared in germ-free environments and lacking commensals, generated accentuated Th2 responses to ovalbumin challenge (636). This effect was reversed when the commensals were replaced. In the human gut, commensals induce Treg responses (624, 637) but whether similar effects occur in the human airway remains unclear. Nevertheless, these findings suggest that commensal bacteria, interacting through the innate immune system, may play a major role in physiologic immune regulation in the upper airway (638).
The nasal and sinus microbiota in CRS has thus far been studied primarily only using conventional techniques. Higher rates of Staphylococcus epidermidis were seen in controls when compared to CRS (639). When ARS and CRS are compared, the majority of reports demonstrate increased rates of S. aureus, gram negative rods and anaerobes in CRS (3, 640-647). Other investigators however, have demonstrated no differences between the nasal bacteriology of CRS and normal controls (648, 649). In cases of unilateral CRS, similar microbiological floras were demonstrated in both diseased and non-diseased sides (650) and culture results did not change after clinically successful sinus surgery (651). Overall, these studies have challenged the role of bacteria in CRS aetiology and pathogenesis, although some of the disparities are likely due to variations in methodology (3), concomitant allergic rhinitis (652), prior antibiotic treatment and source of material for analysis (nasal or sinus). Some investigators have discounted any pathologic role for S. epidermidis, while others do not. The presence of organisms within epithelial cells (653, 654) or in biofilm quora, likely also produces variation in the rate of bacterial identification using conventional techniques. Application of molecular techniques may begin the process of fully defining the nasal microbiota in CRS. Recently, a prospective study of samples obtained from the middle meatus, using the 16S ribosomal DNA technique, revealed a polymicrobial flora in CRS that was distinct from controls (655). Results indicated a preponderance of anaerobes in CRS. Larger studies using metagenomic techniques (656) are likely needed to fully address this issue as the density and composition of the microbial community may play a significant role in regulating host response (624, 657, 658). Specifically, the lower airway microbiota is disordered in asthma and this has been proposed to play a role in disease pathogenesis (630). Whether effects are seen in CRS remains uncertain. Lastly, it should be kept in mind that the vast majority of data has been collected on Caucasian patients in western countries and the bacterial colonization rates in other races and geographic regions may be vastly different in both health and disease (659).
S. aureus is the most common traditional bacterial pathogen identified in CRS patients in western countries (660). The incidence of staphylococcus is much lower in Asian CRSwNP (623) but the presence or absence of bacteria, or any microbial agent, does not mandate or eliminate a role in disease causation. Host evidence of bacteria specific effects does exist for Staphylococcus aureus however, suggesting a role in pathogenesis if not aetiology in at least a subset of CRSwNP patients. Substantial evidence implicating this organism in CRSwNP has accumulated over the last decade, giving rise to the "Staphylococcal Superantigen Hypothesis", which proposes that colonizing S. aureus secretes superantigenic toxins (SAgs) that amplify local eosinophilic inflammation and foster polyp formation (542, 596). In support of this hypothesis, culture studies have indicated a high correlation between the presence of staphylococcus and nasal polyposis (661). These results were supported by the recent demonstration of intracellular S. aureus in CRSwNP, but not in CRSsNP or control patients (607, 608). In addition, approximately 50% of CRSwNP patients demonstrate B and T cells responses in the tissue consistent with prior local staphylococcal superantigen exposure (542, 662-666). These include specific IgE against SAgs as well as clonal proliferation of polyp T cells indicative of local SAg exposure. In addition, SAg toxins have been detected in a portion of CRSwNP patients but not controls (667). These in vivo findings are immunologic 'footprints' of a staphylococcal superantigen effect, which can be demonstrated in approximately 50% of Caucasian nasal polyps as well as a lower percentage of Asian polyps. From a mechanistic standpoint, in vitro studies indicate that SAginduced cytokine release tends to be pro-inflammatory and Th2 skewed, promoting IL-4 and IL-5 but down regulating TGF-β and IL-10 (668-670). SAgs also manipulate eicosanoid metabolism in a pro-inflammatory fashion (605, 606), augment granulocyte migration and survival (671) and furthermore, another staphylococcal toxin (SpA) fosters mast cell degranulation (668). Staphylococcus increased cytokine and MMP expression in polyp and inferior turbinate organ cultures, presumably through a superantigen effect (672). It has also recently been suggested that staphylococcal superantigens may induce glucocorticoid insufficiency through induction of the β isoform of the glucocorticoid receptor (673). Overall, these studies indicate that SAgs have the capacity to foster the Th2 cytokine and remodeling profile observed in Western nasal polyps. Moreover, the demonstration of a local SAg effect correlates with the severity of the eosinophilic inflammation (585). Thus far, there is no evidence of a role for superantigens in CRSsNP, suggesting a distinct aetiology and pathogenesis.
Biofilms and/or intracellular residence of bacteria may increase resistance to standard therapy
In summary, while staphylococcal superantigens appear to amplify and modulate inflammation in nasal polyposis, evidence for a direct aetiologic role is lacking (594). The relatively common intranasal presence of toxigenic staphylococci suggests that unknown host factors likely determine disease expression (674). In addition, approximately 50% of Western polyp patients studied have no evidence of SAg responses yet have a similar phenotypic picture, suggesting that superantigens are not necessary for the typical inflammatory response seen in CRSwNP. Lastly, cystic fibrosis patients exhibit a high rate of staphylococcal colonization and polyp formation yet no evidence of a SAg effect and a strikingly distinct histology and cytokine profile (25). These considerations lead most investigators to view S. aureus as a disease modifier rather than a discrete aetiologic agent but these findings are, nevertheless, molecular evidence indicating staph specific effects (675, 676).
Bacterial biofilms have also been implicated in CRS aetiology and pathogenesis. Biofilms are highly organized structures composed of communities of bacteria encased within a protective extracellular matrix. The formation of bacterial biofilms on surfaces such as the sinonasal mucosa reflects a universal strategy for survival in conditions less than optimal for growth (677, 678). Biofilms serve to protect bacteria from both host defenses and antibiotics (679) and are believed to be a source of recurrent exacerbations in CRS through the periodic release of free-floating bacteria (680). Biofilms are largely absent from controls but have been recovered from both CRSsNP and CRSwNP patients. Reported rates of biofilms in CRS populations vary from 30-100%, likely due to differences in detection methodology (681-690). Multiple bacterial species have been associated with CRS biofilms including H. influenza, S. aureus, S. pneumonia, P. aeruginosa and M. catarrhalis (677, 678, 684, 687, 689). The presence of S. aureus and P. aeruginosa biofilms has been associated with an unfavorable outcome post surgery (686, 691), while the presence of H. influenza biofilms was associated with a favorable outcome and milder disease (692). In particular, S. aureus has been associated with a particularly poor prognosis (693). It has been suggested that S. aureus biofilms foster a Th2 adaptive immune response independent of any staph superantigen effect (603). In contrast, an earlier report demonstrated a shift toward Th1 inflammation in CRS biofilm patients (694). This study was not limited to Staphylococcal biofilms, however. In addition, the differing results may reflect different patient populations in each study, specifically the presence or absence of nasal polyps, rather than intrinsic capabilities of the biofilm to skew the host response. Very recent studies suggest that disruption of the host epithelium may permit biofilm mediated inflammatory effects on the sinonasal tissues (695). Overall, it is widely accepted that biofilms are a bacterial adaptation facilitating resistance to host defenses and antibiotics, helping to foster recalcitrant disease. Moreover, it is also possible that biofilm directed therapies will prove useful in the management of CRS. However, it remains much less clear whether biofilms have any role in the initial establishment of CRS (696).
4.2.3.1. Bacteria
Bacteria have an established role in the aetiology of acute rhinosinusitis (ARS) and it has long been speculated that incompletely treated bacterial ARS leads to the development of CRS. While bacteria may well trigger acute infectious exacerbations, the role of bacteria in the initial establishment of CRS remains unclear. This section will provide an overview of evidence for and against bacteria as aetiologic agents in CRS with emphasis on recent data.
The nasal microbiota is complex and multiple methods, with varying degrees of sensitivity and specificity, have been utilized to determine the bacterial density and composition in health and disease (629). Analysis of samples obtained from the vestibule, measured using molecular techniques, demonstrate multiple bacterial species but a preponderance of the staphylococci and corynebacterium (630, 631). An inverse correlation between the two families was observed, suggesting an antagonistic relationship (632). In addition, the presence of S. epidermidis appears to compete with S. aureus (633). The normal microbiota of the middle meatus may, of course, be quite different than the anterior nostril but these principles likely apply. Healthy sinus cavities, studied using conventional techniques only, appear to have substantially less bacterial colonization than the nasal airway (634). Although not yet tested, more sensitive techniques would likely reveal the presence of a significant bacterial load in the sinuses, given the documented colonization of the lower airway even in healthy individuals (630). Colonizing commensal bacteria in the nose and possibly the sinuses may be important not only in interfering with the growth of pathogens, but also modulating the host immune response (635). This latter effect has been studied in the mouse airway. Animals reared in germ-free environments and lacking commensals, generated accentuated Th2 responses to ovalbumin challenge (636). This effect was reversed when the commensals were replaced. In the human gut, commensals induce Treg responses (624, 637) but whether similar effects occur in the human airway remains unclear. Nevertheless, these findings suggest that commensal bacteria, interacting through the innate immune system, may play a major role in physiologic immune regulation in the upper airway (638).
The nasal and sinus microbiota in CRS has thus far been studied primarily only using conventional techniques. Higher rates of Staphylococcus epidermidis were seen in controls when compared to CRS (639). When ARS and CRS are compared, the majority of reports demonstrate increased rates of S. aureus, gram negative rods and anaerobes in CRS (3, 640-647). Other investigators however, have demonstrated no differences between the nasal bacteriology of CRS and normal controls (648, 649). In cases of unilateral CRS, similar microbiological floras were demonstrated in both diseased and non-diseased sides (650) and culture results did not change after clinically successful sinus surgery (651). Overall, these studies have challenged the role of bacteria in CRS aetiology and pathogenesis, although some of the disparities are likely due to variations in methodology (3), concomitant allergic rhinitis (652), prior antibiotic treatment and source of material for analysis (nasal or sinus). Some investigators have discounted any pathologic role for S. epidermidis, while others do not. The presence of organisms within epithelial cells (653, 654) or in biofilm quora, likely also produces variation in the rate of bacterial identification using conventional techniques. Application of molecular techniques may begin the process of fully defining the nasal microbiota in CRS. Recently, a prospective study of samples obtained from the middle meatus, using the 16S ribosomal DNA technique, revealed a polymicrobial flora in CRS that was distinct from controls (655). Results indicated a preponderance of anaerobes in CRS. Larger studies using metagenomic techniques (656) are likely needed to fully address this issue as the density and composition of the microbial community may play a significant role in regulating host response (624, 657, 658). Specifically, the lower airway microbiota is disordered in asthma and this has been proposed to play a role in disease pathogenesis (630). Whether effects are seen in CRS remains uncertain. Lastly, it should be kept in mind that the vast majority of data has been collected on Caucasian patients in western countries and the bacterial colonization rates in other races and geographic regions may be vastly different in both health and disease (659).
S. aureus is the most common traditional bacterial pathogen identified in CRS patients in western countries (660). The incidence of staphylococcus is much lower in Asian CRSwNP (623) but the presence or absence of bacteria, or any microbial agent, does not mandate or eliminate a role in disease causation. Host evidence of bacteria specific effects does exist for Staphylococcus aureus however, suggesting a role in pathogenesis if not aetiology in at least a subset of CRSwNP patients. Substantial evidence implicating this organism in CRSwNP has accumulated over the last decade, giving rise to the "Staphylococcal Superantigen Hypothesis", which proposes that colonizing S. aureus secretes superantigenic toxins (SAgs) that amplify local eosinophilic inflammation and foster polyp formation (542, 596). In support of this hypothesis, culture studies have indicated a high correlation between the presence of staphylococcus and nasal polyposis (661). These results were supported by the recent demonstration of intracellular S. aureus in CRSwNP, but not in CRSsNP or control patients (607, 608). In addition, approximately 50% of CRSwNP patients demonstrate B and T cells responses in the tissue consistent with prior local staphylococcal superantigen exposure (542, 662-666). These include specific IgE against SAgs as well as clonal proliferation of polyp T cells indicative of local SAg exposure. In addition, SAg toxins have been detected in a portion of CRSwNP patients but not controls (667). These in vivo findings are immunologic 'footprints' of a staphylococcal superantigen effect, which can be demonstrated in approximately 50% of Caucasian nasal polyps as well as a lower percentage of Asian polyps. From a mechanistic standpoint, in vitro studies indicate that SAginduced cytokine release tends to be pro-inflammatory and Th2 skewed, promoting IL-4 and IL-5 but down regulating TGF-β and IL-10 (668-670). SAgs also manipulate eicosanoid metabolism in a pro-inflammatory fashion (605, 606), augment granulocyte migration and survival (671) and furthermore, another staphylococcal toxin (SpA) fosters mast cell degranulation (668). Staphylococcus increased cytokine and MMP expression in polyp and inferior turbinate organ cultures, presumably through a superantigen effect (672). It has also recently been suggested that staphylococcal superantigens may induce glucocorticoid insufficiency through induction of the β isoform of the glucocorticoid receptor (673). Overall, these studies indicate that SAgs have the capacity to foster the Th2 cytokine and remodeling profile observed in Western nasal polyps. Moreover, the demonstration of a local SAg effect correlates with the severity of the eosinophilic inflammation (585). Thus far, there is no evidence of a role for superantigens in CRSsNP, suggesting a distinct aetiology and pathogenesis.
Biofilms and/or intracellular residence of bacteria may increase resistance to standard therapy
In summary, while staphylococcal superantigens appear to amplify and modulate inflammation in nasal polyposis, evidence for a direct aetiologic role is lacking (594). The relatively common intranasal presence of toxigenic staphylococci suggests that unknown host factors likely determine disease expression (674). In addition, approximately 50% of Western polyp patients studied have no evidence of SAg responses yet have a similar phenotypic picture, suggesting that superantigens are not necessary for the typical inflammatory response seen in CRSwNP. Lastly, cystic fibrosis patients exhibit a high rate of staphylococcal colonization and polyp formation yet no evidence of a SAg effect and a strikingly distinct histology and cytokine profile (25). These considerations lead most investigators to view S. aureus as a disease modifier rather than a discrete aetiologic agent but these findings are, nevertheless, molecular evidence indicating staph specific effects (675, 676).
Bacterial biofilms have also been implicated in CRS aetiology and pathogenesis. Biofilms are highly organized structures composed of communities of bacteria encased within a protective extracellular matrix. The formation of bacterial biofilms on surfaces such as the sinonasal mucosa reflects a universal strategy for survival in conditions less than optimal for growth (677, 678). Biofilms serve to protect bacteria from both host defenses and antibiotics (679) and are believed to be a source of recurrent exacerbations in CRS through the periodic release of free-floating bacteria (680). Biofilms are largely absent from controls but have been recovered from both CRSsNP and CRSwNP patients. Reported rates of biofilms in CRS populations vary from 30-100%, likely due to differences in detection methodology (681-690). Multiple bacterial species have been associated with CRS biofilms including H. influenza, S. aureus, S. pneumonia, P. aeruginosa and M. catarrhalis (677, 678, 684, 687, 689). The presence of S. aureus and P. aeruginosa biofilms has been associated with an unfavorable outcome post surgery (686, 691), while the presence of H. influenza biofilms was associated with a favorable outcome and milder disease (692). In particular, S. aureus has been associated with a particularly poor prognosis (693). It has been suggested that S. aureus biofilms foster a Th2 adaptive immune response independent of any staph superantigen effect (603). In contrast, an earlier report demonstrated a shift toward Th1 inflammation in CRS biofilm patients (694). This study was not limited to Staphylococcal biofilms, however. In addition, the differing results may reflect different patient populations in each study, specifically the presence or absence of nasal polyps, rather than intrinsic capabilities of the biofilm to skew the host response. Very recent studies suggest that disruption of the host epithelium may permit biofilm mediated inflammatory effects on the sinonasal tissues (695). Overall, it is widely accepted that biofilms are a bacterial adaptation facilitating resistance to host defenses and antibiotics, helping to foster recalcitrant disease. Moreover, it is also possible that biofilm directed therapies will prove useful in the management of CRS. However, it remains much less clear whether biofilms have any role in the initial establishment of CRS (696).
4.2.3.2. Fungi
The role of fungi in CRS has generated much controversy in the last decade (697, 698). The use of sensitive detection techniques has indicated that fungi are a ubiquitous intranasal presence, identified in close to 100% of both CRS patients and controls (592, 699). As opposed to controls however, patients with CRS also exhibited eosinophils in the nasal tissues and lumen, with no increase in IgE mediated mould allergy (592). These observations formed the basis of the "Fungal Hypothesis of CRS", which proposed that an excessive, non-IgE mediated host response to common airborne fungi is the primary pathogenic trigger in most forms of CRS, both polypoid and non-polypoid, varying only in intensity (593, 700, 701). The primary evidence cited to support this theory was the relative hyper reactivity of peripheral blood mononuclear cells (PBMC) from CRS patients in response to stimulation with supra-physiologic doses of Alternaria antigen in vitro (702). PBMCs from CRS patients expressed significantly higher levels of Th1 and Th2 cytokines upon exposure to Alternaria extract and this heightened response was presumed to reflect an immunologic sensitization of T cells to Alternaria, suggesting it was particularly important in inciting the CRS inflammatory response. As further evidence, nasal mucus or tissue from CRS patients triggered eosinophil migration (703) and a 60-kDa component of the Alternaria fungus was later shown to trigger eosinophil de-granulation via PAR receptor activation in vitro (704). These data were interpreted to suggest that Alternaria served a dual role: first, Alternaria proteins are presented to sensitized T cells inducing a cytokine response that serves to attract and activate eosinophils. Second, Alternaria serves as the target of the eosinophils, triggering de-granulation through a surface PAR receptor, with subsequent mucosal damage. This effector role for eosinophils against fungi was proposed despite the fact that eosinophils do not normally participate to a significant degree in the host defense response targeting fungal organisms (705). Further challenges to the "Fungal Hypothesis" included the observation that the majority of patients in these studies (702, 703) had concomitant asthma, and the heightened cytokine responses from PBMCs as well as the eosinophil migration may reflect priming from this asthma rather than CRS (25, 697, 698). Furthermore, attempts to replicate the fungal-induced cytokine responses from PBMCs by other investigators failed, clearly indicating the absence of a universal hyper-responsiveness to fungal antigens in CRS patients (706, 707). Nevertheless, interest in fungi spawned a series of drug trials using topical intranasal anti-fungal agents that initially provided mixed support for the overall hypothesis (708-711). An extensive, multi-centre, blinded, randomized trial using intra nasal amphotericin failed to show any evidence of efficacy, however (712). More significantly, a follow up study indicated that amphotericin had no significant effect on any pro-inflammatory chemokine, cytokine or growth factor in the CRS lavage samples (713). Overall, the current literature does not support the routine use of topical anti-fungals for CRS (714) and support for the fungal hypothesis as originally proposed is scant.
Evidence for a fungal-specific role in the aetiology of most CRS cases is lacking
The view of fungi as the universal or even primary antigenic stimulus in CRS, has largely faded (715, 716), but this does not eliminate fungi as a factor in CRS aetiology or pathogenesis for at least three reasons. a. Fungi, particularly Alternaria, contain intrinsic proteases that can non-specifically activate protease-activated receptors (PAR) present on the apical surface of nasal epithelial cells with secondary effects on eosinophils and neutrophils (717, 718). Non-specific effects of a protease may be significant, given that epithelial-based protease activated receptors (PAR) are known to be up-regulated in CRS and this signaling may result in significant inflammation in the presence of high levels of fungal organisms (25, 719, 720).
b. Fungi may play a role in the aetiology and pathogenesis of allergic fungal rhinosinusitis (AFRS), classically defined as: (25) nasal polyposis (14) characteristic thick eosinophilic mucin and (594) characteristic CT scan findings (625), type 1 hypersensitivity to fungal antigens by serology or skin tests and (626) fungal elements in the mucin detected by culture or histology (721, 722). AFRS has been proposed to be an immunologically distinct subset of CRS (723). In support of this, peripheral blood mononuclear cells (PBMCs) from AFRS patients were demonstrated to secrete Th2 cytokines in response to fungal antigens (724). In addition to this systemic sensitization, AFRS patients also demonstrate fungalspecific IgE in the eosinophilic mucin (725) and the mucosa (726). The implications of these observations remain far from clear however, as CRSwNP patients with similar, thick eosinophilic mucin but without fungal allergy or gross fungi on histology clearly exist (290). Significantly, the presence or absence of fungal allergy or gross fungi in the eosinophilic mucin had no effect on histology, inflammatory cell infiltrate, tissue eosinophilia or fungal-specific PMBC proliferation (727-729). A small microarray study also showed very few differences in gene expression profiles in the absence of fungi in the mucin (730). The similarities among the groups irrespective of the presence of fungi or fungal allergy have been interpreted by some investigators to indicate that allergy to fungus cannot be the primary pathophysiologic force driving the inflammation in AFRS (79, 731). Further studies will be necessary to resolve the issue (722). c. The cell walls of fungi contain the polysaccharide polymer chitin, which is recognized by pattern recognition receptor(s) in airway epithelial cells triggering innate immune responses (732). Chitin induces a local Th2 immune response in vivo mouse studies, with mucosal infiltration by eosinophils, basophils and Th2 lymphocytes (733). Chitin also induces the enzyme acid mammalian chitinase (AMCase), which acts to degrade the chitin apparently as a defense mechanism, in turn, down regulating the Th2 inflammation (732). AMCase can also be elevated in asthmatic inflammation independent of chitin and in this setting it actually drives Th2 inflammation (732, 734). In the upper airway, epithelial cells also express AMCase and levels are significantly higher in nasal polyps (735-738). Similarly, chitin stimulates AMCase expression by sinonasal epithelial cells in culture (739). While these results are interesting, the clinical significance of chitin or AMCase in lower airway disease remains uncertain, and any role for AMCase or chitin in the etiology or pathogenesis of CRS is even more speculative. In summary, while high levels of fungi may theoretically have direct immuno-stimulatory effects, with the possible exception of AFRS, we lack any consistent in vitro or in vivo evidence demonstrating that fungal antigens are the primary targets of the mucosal T cell or B cell responses observed in CRS. Therefore, despite initial enthusiasm for the fungal hypothesis as the basis for all chronic sinus disease, the current state of basic science evidence coupled with the failure of clinical trials with amphotericin (713), indicates that a central role for fungi in CRS is unlikely.
4.2.3.3. Allergens
The potential role of inhaled allergens in the aetiology and pathogenesis of CRS is controversial, much of which stems from the lack of uniform definitions of both CRS and atopy, variability in allergy testing methodologies and potential referral bias in patients receiving allergy testing (79, 740). From a pathophysiological standpoint, allergic rhinitis (AR) occurs through host sensitization to antigenic foreign protein across a mucosal barrier via dendritic cells and naive CD4+ lymphocytes, with the generation of antigen specific Th2 lymphocytes and IgE secreting plasma cells. Subsequent antigenic challenge across the mucosa results in cross linking of IgE bound to the surface of mast cells and subsequent de-granulation as well as the release of additional Th2 cytokines leading to recruitment of inflammatory cells including eosinophils. The pathogenesis of CRS is much less clear but at least some of these mechanisms are operative. Clinically, the symptoms of AR also overlap with CRS to a substantial degree (26) but are generally less severe than those present in most forms of CRS. Studies in CRS indicate that inflammation in the sinus mucosa and nasal mucosa are similar in profile if not disease intensity establishing the term 'rhinosinusitis' (14). Recent studies have indicated that nasal challenge with allergen leads to secondary maxillary sinus inflammation (741). This is in accordance with reports demonstrating CT changes induced in ragweed allergic rhinitis (742). Technically speaking then, AR could actually be termed allergic rhinosinusitis exhibiting not only nasal, but also chronic sinus mucosal inflammation, at least in more severe circumstances such as perennial AR, which has a markedly more intense inflammatory profile than intermittent AR (743). Hence, perennial AR could be included under the CRS definition: inflammation of the nasal and sinus mucosa of over 12 weeks duration. From this perspective, much of the confusion in regard the role of AR in CRS becomes clear. AR can be viewed as just one mechanism of sinonasal mucosal inflammation, that is comparatively well understood from a molecular perspective, sharing the same effector cells, cytokines and inflammatory mediators active in many forms of CRS. The contribution of AR to the total inflammatory picture in CRS is typically relatively mild however, since the presence of allergic rhinitis (as defined by positive RAST or skin testing) did not influence symptom severity, extent of disease on CT scan or likelihood of surgical failure when compared to non-allergic CRS (744-746). Furthermore, avoidance and immunotherapy relieved some associated rhinitis symptoms but did not reverse sinonasal disease (79).
In summary then, while more severe, perennial forms of allergic rhinitis might technically fulfill the definition of CRS, evidence is weak in support of a role for AR in the aetiology of the typical case of CRS. The most reasonable conclusion appears to be that AR should generally be considered a superimposed problem, which contributes in a variable but relatively mild way to the sinonasal inflammation seen in most CRS patients. Notable potential exceptions may be the patients with severe CRSwNP associated with (25) multiple positive skin tests, suggesting a generalized barrier failure (14, 23) allergic fungal rhinosinusitis (722) as discussed above and (594) patients with local polyclonal IgE in the absence of systemic atopy (542, 596). It has been suggested that this subgroup of patients manifests a superantigen driven local polyclonal IgE response to a diffuse array of environmental agents with resultant massive chronic mast cell stimulation (747).
4.2.3.4. Viruses
The defense against respiratory viruses involves both innate and adaptive immunity (748). These protective responses trigger sinus inflammation demonstrable on CT scans but the effects are presumed to be transient (749) and despite the frequency of viral upper respiratory infections (URIs), relatively little attention has been paid to any association with CRS. In assessing a role for viruses in the aetiology and pathogenesis of CRS, the topic will be divided into 3 hypotheses:
With regard to exacerbations of airway disease, viral infections have been clearly implicated in exacerbations of asthma and COPD (754-757). Viral URIs are also presumed to precede most episodes of acute bacterial rhinosinusitis. With regard to CRS exacerbations, in vivo data is lacking but it has been proposed that viral infection in combination with cigarette smoke fosters epithelial activation contributing to acute exacerbations of CRS (758). These in vitro studies using double stranded RNA plus cigarette smoke triggered increased RANTES expression in nasal epithelial cells, which should foster an eosinophilic response in vivo. In summary, the potential relationship between viral infection and CRS is relatively unstudied. Nevertheless, given the documented ability of viral upper respiratory infections to disrupt the upper airway epithelial barrier (759), it is clearly possible they play a role in the aetiology and pathogenesis of CRS.
4.2.3.5. Environmental Toxins
Exposure to toxins such as tobacco smoke, ozone, sulphur dioxide, nitrogen dioxide and particulate air pollutants (e.g. diesel exhaust fumes) have the potential to trigger damage to the epithelium and, in principle, accentuate airway inflammation. These agents induce oxidative and nitrosative stress with production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) that have the capacity to cause tissue damage (759). The significance of most toxin exposures in CRS is unclear, although a number of studies have focussed on the effects of tobacco smoke. The prevalence of CRS has been reported to be higher in smokers (760, 761) and smokers have a less favorable response to surgery (762, 763). The deleterious effects of cigarette smoke relevant to CRS include alterations in secretion and ciliary beat frequency (764) as well the induction of bacterial biofilms (765). Based on in vitro data, it has been proposed that cigarette smoke in combination with viral infection contributes to acute exacerbations and eosinophilic inflammation in CRS patients (758). ROS and RNS from tobacco smoke induces proinflammatory cytokine secretion (765), epithelial apoptosis (766, 767) and diminished airway epithelial barrier function (768). Overall, data suggest that cigarette smoke likely can contribute to the inflammation in CRS in exposed individuals, but evidence for a role in the initial establishment of the disorder is lacking. In particular, a recent study has suggested that in contrast to the lower airways, the pro-inflammatory effects of tobacco smoke in the upper airway appear to be down-regulated over time (769). Outcome studies have also failed to show a strong negative effect from smoking (770). These findings would argue against a significant role for tobacco smoke in CRS aetiology.
The role of fungi in CRS has generated much controversy in the last decade (697, 698). The use of sensitive detection techniques has indicated that fungi are a ubiquitous intranasal presence, identified in close to 100% of both CRS patients and controls (592, 699). As opposed to controls however, patients with CRS also exhibited eosinophils in the nasal tissues and lumen, with no increase in IgE mediated mould allergy (592). These observations formed the basis of the "Fungal Hypothesis of CRS", which proposed that an excessive, non-IgE mediated host response to common airborne fungi is the primary pathogenic trigger in most forms of CRS, both polypoid and non-polypoid, varying only in intensity (593, 700, 701). The primary evidence cited to support this theory was the relative hyper reactivity of peripheral blood mononuclear cells (PBMC) from CRS patients in response to stimulation with supra-physiologic doses of Alternaria antigen in vitro (702). PBMCs from CRS patients expressed significantly higher levels of Th1 and Th2 cytokines upon exposure to Alternaria extract and this heightened response was presumed to reflect an immunologic sensitization of T cells to Alternaria, suggesting it was particularly important in inciting the CRS inflammatory response. As further evidence, nasal mucus or tissue from CRS patients triggered eosinophil migration (703) and a 60-kDa component of the Alternaria fungus was later shown to trigger eosinophil de-granulation via PAR receptor activation in vitro (704). These data were interpreted to suggest that Alternaria served a dual role: first, Alternaria proteins are presented to sensitized T cells inducing a cytokine response that serves to attract and activate eosinophils. Second, Alternaria serves as the target of the eosinophils, triggering de-granulation through a surface PAR receptor, with subsequent mucosal damage. This effector role for eosinophils against fungi was proposed despite the fact that eosinophils do not normally participate to a significant degree in the host defense response targeting fungal organisms (705). Further challenges to the "Fungal Hypothesis" included the observation that the majority of patients in these studies (702, 703) had concomitant asthma, and the heightened cytokine responses from PBMCs as well as the eosinophil migration may reflect priming from this asthma rather than CRS (25, 697, 698). Furthermore, attempts to replicate the fungal-induced cytokine responses from PBMCs by other investigators failed, clearly indicating the absence of a universal hyper-responsiveness to fungal antigens in CRS patients (706, 707). Nevertheless, interest in fungi spawned a series of drug trials using topical intranasal anti-fungal agents that initially provided mixed support for the overall hypothesis (708-711). An extensive, multi-centre, blinded, randomized trial using intra nasal amphotericin failed to show any evidence of efficacy, however (712). More significantly, a follow up study indicated that amphotericin had no significant effect on any pro-inflammatory chemokine, cytokine or growth factor in the CRS lavage samples (713). Overall, the current literature does not support the routine use of topical anti-fungals for CRS (714) and support for the fungal hypothesis as originally proposed is scant.
Evidence for a fungal-specific role in the aetiology of most CRS cases is lacking
The view of fungi as the universal or even primary antigenic stimulus in CRS, has largely faded (715, 716), but this does not eliminate fungi as a factor in CRS aetiology or pathogenesis for at least three reasons. a. Fungi, particularly Alternaria, contain intrinsic proteases that can non-specifically activate protease-activated receptors (PAR) present on the apical surface of nasal epithelial cells with secondary effects on eosinophils and neutrophils (717, 718). Non-specific effects of a protease may be significant, given that epithelial-based protease activated receptors (PAR) are known to be up-regulated in CRS and this signaling may result in significant inflammation in the presence of high levels of fungal organisms (25, 719, 720).
b. Fungi may play a role in the aetiology and pathogenesis of allergic fungal rhinosinusitis (AFRS), classically defined as: (25) nasal polyposis (14) characteristic thick eosinophilic mucin and (594) characteristic CT scan findings (625), type 1 hypersensitivity to fungal antigens by serology or skin tests and (626) fungal elements in the mucin detected by culture or histology (721, 722). AFRS has been proposed to be an immunologically distinct subset of CRS (723). In support of this, peripheral blood mononuclear cells (PBMCs) from AFRS patients were demonstrated to secrete Th2 cytokines in response to fungal antigens (724). In addition to this systemic sensitization, AFRS patients also demonstrate fungalspecific IgE in the eosinophilic mucin (725) and the mucosa (726). The implications of these observations remain far from clear however, as CRSwNP patients with similar, thick eosinophilic mucin but without fungal allergy or gross fungi on histology clearly exist (290). Significantly, the presence or absence of fungal allergy or gross fungi in the eosinophilic mucin had no effect on histology, inflammatory cell infiltrate, tissue eosinophilia or fungal-specific PMBC proliferation (727-729). A small microarray study also showed very few differences in gene expression profiles in the absence of fungi in the mucin (730). The similarities among the groups irrespective of the presence of fungi or fungal allergy have been interpreted by some investigators to indicate that allergy to fungus cannot be the primary pathophysiologic force driving the inflammation in AFRS (79, 731). Further studies will be necessary to resolve the issue (722). c. The cell walls of fungi contain the polysaccharide polymer chitin, which is recognized by pattern recognition receptor(s) in airway epithelial cells triggering innate immune responses (732). Chitin induces a local Th2 immune response in vivo mouse studies, with mucosal infiltration by eosinophils, basophils and Th2 lymphocytes (733). Chitin also induces the enzyme acid mammalian chitinase (AMCase), which acts to degrade the chitin apparently as a defense mechanism, in turn, down regulating the Th2 inflammation (732). AMCase can also be elevated in asthmatic inflammation independent of chitin and in this setting it actually drives Th2 inflammation (732, 734). In the upper airway, epithelial cells also express AMCase and levels are significantly higher in nasal polyps (735-738). Similarly, chitin stimulates AMCase expression by sinonasal epithelial cells in culture (739). While these results are interesting, the clinical significance of chitin or AMCase in lower airway disease remains uncertain, and any role for AMCase or chitin in the etiology or pathogenesis of CRS is even more speculative. In summary, while high levels of fungi may theoretically have direct immuno-stimulatory effects, with the possible exception of AFRS, we lack any consistent in vitro or in vivo evidence demonstrating that fungal antigens are the primary targets of the mucosal T cell or B cell responses observed in CRS. Therefore, despite initial enthusiasm for the fungal hypothesis as the basis for all chronic sinus disease, the current state of basic science evidence coupled with the failure of clinical trials with amphotericin (713), indicates that a central role for fungi in CRS is unlikely.
4.2.3.3. Allergens
The potential role of inhaled allergens in the aetiology and pathogenesis of CRS is controversial, much of which stems from the lack of uniform definitions of both CRS and atopy, variability in allergy testing methodologies and potential referral bias in patients receiving allergy testing (79, 740). From a pathophysiological standpoint, allergic rhinitis (AR) occurs through host sensitization to antigenic foreign protein across a mucosal barrier via dendritic cells and naive CD4+ lymphocytes, with the generation of antigen specific Th2 lymphocytes and IgE secreting plasma cells. Subsequent antigenic challenge across the mucosa results in cross linking of IgE bound to the surface of mast cells and subsequent de-granulation as well as the release of additional Th2 cytokines leading to recruitment of inflammatory cells including eosinophils. The pathogenesis of CRS is much less clear but at least some of these mechanisms are operative. Clinically, the symptoms of AR also overlap with CRS to a substantial degree (26) but are generally less severe than those present in most forms of CRS. Studies in CRS indicate that inflammation in the sinus mucosa and nasal mucosa are similar in profile if not disease intensity establishing the term 'rhinosinusitis' (14). Recent studies have indicated that nasal challenge with allergen leads to secondary maxillary sinus inflammation (741). This is in accordance with reports demonstrating CT changes induced in ragweed allergic rhinitis (742). Technically speaking then, AR could actually be termed allergic rhinosinusitis exhibiting not only nasal, but also chronic sinus mucosal inflammation, at least in more severe circumstances such as perennial AR, which has a markedly more intense inflammatory profile than intermittent AR (743). Hence, perennial AR could be included under the CRS definition: inflammation of the nasal and sinus mucosa of over 12 weeks duration. From this perspective, much of the confusion in regard the role of AR in CRS becomes clear. AR can be viewed as just one mechanism of sinonasal mucosal inflammation, that is comparatively well understood from a molecular perspective, sharing the same effector cells, cytokines and inflammatory mediators active in many forms of CRS. The contribution of AR to the total inflammatory picture in CRS is typically relatively mild however, since the presence of allergic rhinitis (as defined by positive RAST or skin testing) did not influence symptom severity, extent of disease on CT scan or likelihood of surgical failure when compared to non-allergic CRS (744-746). Furthermore, avoidance and immunotherapy relieved some associated rhinitis symptoms but did not reverse sinonasal disease (79).
In summary then, while more severe, perennial forms of allergic rhinitis might technically fulfill the definition of CRS, evidence is weak in support of a role for AR in the aetiology of the typical case of CRS. The most reasonable conclusion appears to be that AR should generally be considered a superimposed problem, which contributes in a variable but relatively mild way to the sinonasal inflammation seen in most CRS patients. Notable potential exceptions may be the patients with severe CRSwNP associated with (25) multiple positive skin tests, suggesting a generalized barrier failure (14, 23) allergic fungal rhinosinusitis (722) as discussed above and (594) patients with local polyclonal IgE in the absence of systemic atopy (542, 596). It has been suggested that this subgroup of patients manifests a superantigen driven local polyclonal IgE response to a diffuse array of environmental agents with resultant massive chronic mast cell stimulation (747).
4.2.3.4. Viruses
The defense against respiratory viruses involves both innate and adaptive immunity (748). These protective responses trigger sinus inflammation demonstrable on CT scans but the effects are presumed to be transient (749) and despite the frequency of viral upper respiratory infections (URIs), relatively little attention has been paid to any association with CRS. In assessing a role for viruses in the aetiology and pathogenesis of CRS, the topic will be divided into 3 hypotheses:
- viruses are a chronic source of mucosal inflammation
-
viruses trigger the initial insult that pre-disposes to CRS
and
-
viruses trigger acute exacerbations of CRS (750).
With regard to exacerbations of airway disease, viral infections have been clearly implicated in exacerbations of asthma and COPD (754-757). Viral URIs are also presumed to precede most episodes of acute bacterial rhinosinusitis. With regard to CRS exacerbations, in vivo data is lacking but it has been proposed that viral infection in combination with cigarette smoke fosters epithelial activation contributing to acute exacerbations of CRS (758). These in vitro studies using double stranded RNA plus cigarette smoke triggered increased RANTES expression in nasal epithelial cells, which should foster an eosinophilic response in vivo. In summary, the potential relationship between viral infection and CRS is relatively unstudied. Nevertheless, given the documented ability of viral upper respiratory infections to disrupt the upper airway epithelial barrier (759), it is clearly possible they play a role in the aetiology and pathogenesis of CRS.
4.2.3.5. Environmental Toxins
Exposure to toxins such as tobacco smoke, ozone, sulphur dioxide, nitrogen dioxide and particulate air pollutants (e.g. diesel exhaust fumes) have the potential to trigger damage to the epithelium and, in principle, accentuate airway inflammation. These agents induce oxidative and nitrosative stress with production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) that have the capacity to cause tissue damage (759). The significance of most toxin exposures in CRS is unclear, although a number of studies have focussed on the effects of tobacco smoke. The prevalence of CRS has been reported to be higher in smokers (760, 761) and smokers have a less favorable response to surgery (762, 763). The deleterious effects of cigarette smoke relevant to CRS include alterations in secretion and ciliary beat frequency (764) as well the induction of bacterial biofilms (765). Based on in vitro data, it has been proposed that cigarette smoke in combination with viral infection contributes to acute exacerbations and eosinophilic inflammation in CRS patients (758). ROS and RNS from tobacco smoke induces proinflammatory cytokine secretion (765), epithelial apoptosis (766, 767) and diminished airway epithelial barrier function (768). Overall, data suggest that cigarette smoke likely can contribute to the inflammation in CRS in exposed individuals, but evidence for a role in the initial establishment of the disorder is lacking. In particular, a recent study has suggested that in contrast to the lower airways, the pro-inflammatory effects of tobacco smoke in the upper airway appear to be down-regulated over time (769). Outcome studies have also failed to show a strong negative effect from smoking (770). These findings would argue against a significant role for tobacco smoke in CRS aetiology.
4.2.4. Host Inflammatory Pathways in CRS
Sinonasal mucosa serves as the site of interface with inhaled irritants, aero-allergens, commensal organisms and pathogens. Mucociliary clearance, physical exclusion, and the innate and adaptive immune responses all serve as a barrier, protecting host from environment. The major environmental agents thus far implicated in CRS were discussed in the preceeding segment but their pathophysiologic importance remains unclear. In the normal patient, these common entities are cleared without tissue damage or the establishment of a chronic process. It has been proposed that alterations in the host mucosal innate immune response may predispose to the development of CRS (25, 597). This hypothesis shifts emphasis away from identifying singular environmental or microbial agents and implicates host susceptibility as the major factor in CRS pathogenesis. A recent expert panel has gone further, proposing the question whether all CRS patients may be immunodeficient in some fashion (628). If correct, it should be kept in mind that the majority of idiopathic CRS patients do not suffer from chronic inflammatory pathology outside of the airway. The association of asthma and CRS is well established, but the prevalence of other chronic inflammatory disorders in the CRS population was not found to be significantly above background (771). These observations suggest the corollary hypothesis: immune abnormalities, if present in CRS, will be mediated by processes centred in the airway mucosa. Regardless of the ultimate validity of these concepts, the mucosal inflammation in CRS is highly variable in character, an observation predictable given the broad definition of this entity. Currently, the most widely accepted sub types of idiopathic CRS are the forms with and without nasal polyps, as the gross finding of ballooned mucosa suggests a distinct pathway or pathways in this subset of patients. These two groups are themselves heterogeneous, however, and incompletely characterized from a standpoint of pathogenesis. Distinguishing the molecular pathways that characterise or underlie CRS inflammation should be of value in determining pathophysiology, further defining sub types of CRS and ultimately, guiding new therapeutic approaches (628). The following segment will review the current literature on the various components of the sinonasal mucosal defense system, with emphasis on areas relevant to CRS.
4.2.4.1. Mechanical Barrier
The mechanical barrier of the sinonasal mucosa consists of the mucus, motile cilia and respiratory epithelial cells linked by adhesion complexes that include apical tight junctions. Mucociliary transport is the first line of defense, trapping foreign material in the mucus blanket and moving it out of the sinuses and nasal cavity towards the nasopharynx. The source of nasal secretions includes submucosal glands, goblet cells, epithelial cell proteins, lacrimal secretion and vascular transudate. Respiratory mucus includes a low viscosity inner sol layer and a high viscosity outer gel layer, which rides along the tips of the extended cilia. The major protein components of respiratory secretions are the mucin glycoproteins with peptide backbones and oligosaccharide side chains; these glycoproteins likely play a significant role in organizing the mucus, secondarily influencing host-microbial interactions (772). In addition, mucins bind surface adhesins on microorganisms limiting their ability to access the epithelium and facilitating mucociliary transport out of the nasal cavity (773). The relevance of this process to CRS is underscored by the high prevalence of sinonasal inflammation observed in patients with gene defects affecting mucociliary flow such as cystic fibrosis (chloride transport) and Kartagener's syndrome (ciliary dyskinesia) (773). Individuals that are heterozygous for CFTR mutations are also more likely to suffer from CRS (774). Furthermore, in idiopathic CRS there is evidence for ciliary dysfunction in explanted epithelial cells (775). Clinically, increased mucus viscosity correlates with disease severity in CRS (776) and drugs that reduce viscosity have been proposed as therapeutic agents (777-779).
Host defects in the mechanical barrier, mucociliary flow and the innate immunity (e.g. lactoferrin and S100 proteins) have been associated with CRS
Breakdown of the mechanical components of an epithelial barrier can play an important role in permitting foreign proteins to stimulate an immune response and this has been proposed as a major factor in the aetiology of asthma (768). Airway epithelial cells are linked by an apical intercellular adhesion complex composed of tight junctions, intermediate junctions, desmosomes and hemidesmisomes. In CRSwNP, significantly decreased levels of the desmosomal proteins DSG2 and DSG3 (780) and tight junction proteins claudin and occludin (781) have been reported. Expression of the epithelial protein LEKT1 is also significantly decreased in CRSwNP (782). This protein, encoded by the gene SPINK5, acts as a protease inhibitor involved in regulating the processing of the tight junction proteins critical to epithelial barrier function in the skin. Interestingly, mutations in SPINK5 are shown to be responsible for Netherton syndrome- a rare autosomal recessive condition that results in flaky skin, fragile hair and severe atopy (783). Lower levels of protease inhibitors like LEKT1 in CRS epithelium may result in increased susceptibility to endogenous and exogenous protease activity (597). Fungi, bacteria and many allergens all possess significant intrinsic protease activity, which, in the presence of deficient endogenous protease inhibitors such as LEKT1, may render the mechanical barrier more vulnerable to protease attack and greater mucosal penetration of foreign proteins. Further functional evidence for barrier dysfunction in CRS is demonstrated by higher rates of ion permeability in cultured epithelial monolayers derived from CRS patients when compared with normal controls (784). Increased ion transport has been proposed as a mechanism for tissue oedema seen in nasal polyps (785, 786).
Taken together, these studies suggest that defective mucociliary function may play a role in the pathogenesis of CRS broadly, while mechanical barrier disruption has been more closely linked to CRSwNP.
Sinonasal mucosa serves as the site of interface with inhaled irritants, aero-allergens, commensal organisms and pathogens. Mucociliary clearance, physical exclusion, and the innate and adaptive immune responses all serve as a barrier, protecting host from environment. The major environmental agents thus far implicated in CRS were discussed in the preceeding segment but their pathophysiologic importance remains unclear. In the normal patient, these common entities are cleared without tissue damage or the establishment of a chronic process. It has been proposed that alterations in the host mucosal innate immune response may predispose to the development of CRS (25, 597). This hypothesis shifts emphasis away from identifying singular environmental or microbial agents and implicates host susceptibility as the major factor in CRS pathogenesis. A recent expert panel has gone further, proposing the question whether all CRS patients may be immunodeficient in some fashion (628). If correct, it should be kept in mind that the majority of idiopathic CRS patients do not suffer from chronic inflammatory pathology outside of the airway. The association of asthma and CRS is well established, but the prevalence of other chronic inflammatory disorders in the CRS population was not found to be significantly above background (771). These observations suggest the corollary hypothesis: immune abnormalities, if present in CRS, will be mediated by processes centred in the airway mucosa. Regardless of the ultimate validity of these concepts, the mucosal inflammation in CRS is highly variable in character, an observation predictable given the broad definition of this entity. Currently, the most widely accepted sub types of idiopathic CRS are the forms with and without nasal polyps, as the gross finding of ballooned mucosa suggests a distinct pathway or pathways in this subset of patients. These two groups are themselves heterogeneous, however, and incompletely characterized from a standpoint of pathogenesis. Distinguishing the molecular pathways that characterise or underlie CRS inflammation should be of value in determining pathophysiology, further defining sub types of CRS and ultimately, guiding new therapeutic approaches (628). The following segment will review the current literature on the various components of the sinonasal mucosal defense system, with emphasis on areas relevant to CRS.
4.2.4.1. Mechanical Barrier
The mechanical barrier of the sinonasal mucosa consists of the mucus, motile cilia and respiratory epithelial cells linked by adhesion complexes that include apical tight junctions. Mucociliary transport is the first line of defense, trapping foreign material in the mucus blanket and moving it out of the sinuses and nasal cavity towards the nasopharynx. The source of nasal secretions includes submucosal glands, goblet cells, epithelial cell proteins, lacrimal secretion and vascular transudate. Respiratory mucus includes a low viscosity inner sol layer and a high viscosity outer gel layer, which rides along the tips of the extended cilia. The major protein components of respiratory secretions are the mucin glycoproteins with peptide backbones and oligosaccharide side chains; these glycoproteins likely play a significant role in organizing the mucus, secondarily influencing host-microbial interactions (772). In addition, mucins bind surface adhesins on microorganisms limiting their ability to access the epithelium and facilitating mucociliary transport out of the nasal cavity (773). The relevance of this process to CRS is underscored by the high prevalence of sinonasal inflammation observed in patients with gene defects affecting mucociliary flow such as cystic fibrosis (chloride transport) and Kartagener's syndrome (ciliary dyskinesia) (773). Individuals that are heterozygous for CFTR mutations are also more likely to suffer from CRS (774). Furthermore, in idiopathic CRS there is evidence for ciliary dysfunction in explanted epithelial cells (775). Clinically, increased mucus viscosity correlates with disease severity in CRS (776) and drugs that reduce viscosity have been proposed as therapeutic agents (777-779).
Host defects in the mechanical barrier, mucociliary flow and the innate immunity (e.g. lactoferrin and S100 proteins) have been associated with CRS
Breakdown of the mechanical components of an epithelial barrier can play an important role in permitting foreign proteins to stimulate an immune response and this has been proposed as a major factor in the aetiology of asthma (768). Airway epithelial cells are linked by an apical intercellular adhesion complex composed of tight junctions, intermediate junctions, desmosomes and hemidesmisomes. In CRSwNP, significantly decreased levels of the desmosomal proteins DSG2 and DSG3 (780) and tight junction proteins claudin and occludin (781) have been reported. Expression of the epithelial protein LEKT1 is also significantly decreased in CRSwNP (782). This protein, encoded by the gene SPINK5, acts as a protease inhibitor involved in regulating the processing of the tight junction proteins critical to epithelial barrier function in the skin. Interestingly, mutations in SPINK5 are shown to be responsible for Netherton syndrome- a rare autosomal recessive condition that results in flaky skin, fragile hair and severe atopy (783). Lower levels of protease inhibitors like LEKT1 in CRS epithelium may result in increased susceptibility to endogenous and exogenous protease activity (597). Fungi, bacteria and many allergens all possess significant intrinsic protease activity, which, in the presence of deficient endogenous protease inhibitors such as LEKT1, may render the mechanical barrier more vulnerable to protease attack and greater mucosal penetration of foreign proteins. Further functional evidence for barrier dysfunction in CRS is demonstrated by higher rates of ion permeability in cultured epithelial monolayers derived from CRS patients when compared with normal controls (784). Increased ion transport has been proposed as a mechanism for tissue oedema seen in nasal polyps (785, 786).
Taken together, these studies suggest that defective mucociliary function may play a role in the pathogenesis of CRS broadly, while mechanical barrier disruption has been more closely linked to CRSwNP.
4.2.4.2. Epithelial Cells
4.2.4.2.1. Receptors
In addition to the physical barrier, sinonasal epithelial cells (ECs) play an active role in both the innate and acquired immune response (787, 788). Membrane bound and cytoplasmic pattern recognition receptors (PRRs) that recognize pathogen associated molecular patterns (PAMPs) have been identified on airway epithelial cells (139, 159, 789-791). PAMPs are conserved molecular patterns found in parasites, viruses, yeasts, bacteria and mycobacteria; recognition by host epithelial cells through PRRs results in the release of innate protective agents as well as chemokines and cytokines that attract innate cellular defenses (e.g. neutrophils). In addition to PAMPs, cells also sense cellular damage through damage-associated molecular patterns (DAMPs) (159, 792). The combined signal of foreign material plus cellular damage triggers an innate response and sets in motion, and ultimately helps determine the nature of, the adaptive immune response (793).
Prominent amongst the PRRs are the Toll-like receptors (TLRs), currently a family of 10 integral membrane glycoproteins that recognize extracellular or intracellular PAMPs such as bacterial cell-surface lipopolysaccharides (LPS) or unmethylated CpG islands found in pathogen DNA. Engagement of the TLRs by a PAMP triggers intracellular signaling through adapter proteins like MyD88 or TRIF that in turn can effect pro-inflammatory gene expression through the activation of nuclear transcription factors such as NF-kB, AP-1 and IRF3 (794). Given that TLR2, TLR3, TLR4 and TLR9 in particular are expressed on airway epithelium, it is likely they play an important role in mediating host inflammation, with potential derangements contributing to the development of CRS (795). This hypothesis is supported by the quantitative increase in TLR2 mRNA seen in cystic fibrosis polyps and in some studies of CRSsNP, (796, 797), as well as reported decreases in mucosal TLR2 and TLR9 mRNA in samples from CRSwNP (791, 798). These results have not been confirmed at the protein level nor is there data demonstrating a functional deficit of TLR signaling in CRS patients. Nevertheless, this remains a theoretical mechanism that can account for chronic mucosal inflammation and merits further exploration. Data regarding dysregulation of other PRRs in CRS is sparse, although NOD-like receptors (NLRs) are expressed in nasal and sinus epithelial cells (799). A single study indicated that levels were increased in CRSwNP epithelium and this level was decreased after nasal steroid use (800).
In addition to PRRs, sinonasal epithelial cells also express protease-activated receptors (PAR) (720). Although not classically considered host defense molecules, these receptors are activated by a variety of endogenous and exogenous proteases, including those associated with bacteria, fungi and allergens (801). Triggering of PAR receptors invokes the NFκβ signaling pathway that results in cytokine and chemokine production, cellular recruitment and potentially, skewing of the both the innate and acquired immune response (794, 802). In vitro studies on the nasal epithelium have indicated that PAR-2 activation triggers IL-8 release and this response can be elicited by Staphylococcus aureus-derived proteases (720, 803). Other investigators have suggested that fungal proteases may act on PAR receptors to drive both a neutrophilic and eosinophilic response (718). As mentioned earlier LEKT1 protein, a natural anti-protease, is reduced in CRSwNP epithelium (782). In addition to protecting tight junctions discussed above, this protein should also serve to shield epithelialbased PAR receptors. In a model of skin disease, a recent study demonstrated that the absence of LEKT1 leads to the expression of the Th2 skewing molecule TSLP via activation of PAR-2 (804). It has been suggested that LEKT deficiency may contribute to the pathogenesis of CRS via inappropriate PAR stimulation as well (597). This may be of particular significance given that CRSsNP and CRSwNP both express higher levels of PAR2 in comparison to normal ECs (720).
4.2.4.2.2. Epithelial Cell Response: Host defense molecules
Epithelial cells secrete a vast arsenal of antimicrobial molecules in several classes including enzymes (lysozyme, chitinases and peroxidases), opsonins (complement and pentraxin-3), permeabilizing proteins (A defensins, B defensins and cathelicidins such as LL-37), collectins (surfactant protein-A, surfactant protein-D and mannosebinding lectin) and binding proteins (lactoferrin and mucins) (611, 805, 806). Studies of CRS patients have not demonstrated a universal trend in the expression of these antimicrobial molecules. Levels for complement components, LL-37, surfactant protein A (SP-A) and Acid Mammalian Chitinase demonstrated increases, presumably compensatory, in CRS patients (735, 807-811). Lactoferrin and the S100 group of antimicrobials were decreased in CRS, however (686, 812, 813). The S100 proteins are products of a multi-gene family widely expressed in epithelial cells. In addition to direct anti-microbial effects, these have diverse effects on cell differentiation and wound healing, linking the mechanical barrier and classic anti-microbial properties (814). PLUNC (Palate Lung Nasal Epithelial Clone), another secreted antimicrobial protein, is decreased in CRSwNP (815). PLUNC is secreted by glandular rather than surface epithelium and this protein may have particular relevance for CRS as it possesses anti-biofilm properties.
Sinonasal epithelial cells (ECs) play an active role in both the innate and acquired immune response
Presence of diminished host defense molecules in CRS suggests the hypothesis that a primary sinonasal innate immune defect may contribute to local microbial proliferation and the development of CRS in a subset of patients (597). There is some evidence however that Th2 cytokines can cause nasal epithelial cells to down regulate the production of innate immune molecules such as human beta-defensin 2 and surfactant protein A (816). This suggests the alternative hypothesis that an inappropriate Th2 effector response at the mucosal surface may account for the observed innate immune deficiencies. Mechanistic studies to uncover whether diminished EC innate immune responses in CRS are constitutive and pre-exist the onset of CRS or are inducible responses to Th2 inflammation are still incomplete. Nevertheless, innate immune responses in ECs can be induced by the T cell cytokine IL-22, which works through its receptor IL-22R (817, 818). Binding of IL-22 to its receptor activates the transcription factor STAT 3, which mediates mucosal host defense and epithelial repair (819, 820). In airway ECs, the STAT 3 pathway regulates production of host defense molecules including the S100 family (821). Studies in the gut and lung indicate that this pathway is critical in the regulation of inflammatory responses at the epithelial surface in general (822). In CRSwNP, diminished expression of IL-22R has been reported (823) and separate studies have indicated that the STAT 3 pathway is blunted in CRSwNP (598). Interestingly, STAT 3 mutations have been indentified in Hyper IgE syndrome (HIES or Job's syndrome), which is associated with eosinophilia, high IgE, staph abscesses and recurrent sino-pulmonary infections (599). The similarities between CRSwNP and some aspects of Job's syndrome suggest the hypothesis that nasal polyposis may result from local (sinonasal) impairment of the STAT 3 pathway (598).
4.2.4.2.3. Epithelial Cell Response: Cytokines and Chemokines
Airway epithelial cells produce a variety of inflammatory cytokines, typically in response to PRR and PAR receptor stimulation (794). A partial list includes IL-1, TNF-α, IFNα/β, GM-CSF, eotaxins, RANTES, IP-10, IL-6, IL-8, GRO-α, MDC, SCF, TARC, MCP-4, BAFF, osteopontin, IL-25, IL-32, IL-33 and TSLP (24, 600, 805, 824-826). In addition to driving pain, swelling, vascular dilation and leak and other hallmarks of inflammation, many of these cytokines have chemokine properties attracting various leukocytes including eosinophils, mast cells, neutrophils, dendritic cells and lymphocytes. EC cytokines are also believed to play a key role in dendritic cell polarization, shaping the nature of the T cell response to antigens (827). Given the important role of ECs in mucosal immunity, altered nasal EC cytokine release may play a role in CRS pathogenesis. The contribution of EC gene expression to the overall mucosal cytokine milieu can be difficult to determine. Quantitative cytokine studies in CRS have been done on whole tissue biopsies for the most part, as the techniques needed to analyze isolated nasal EC secretion are more problematic. In vivo epithelial scrapings and in vitro EC cultures both have limitations: the former is generally limited to mRNA analysis as adequate protein samples are difficult to obtain while the latter is technically difficult and prone to potential cell culture effects. In vitro studies have most commonly demonstrated elevated EC cytokine secretion from CRS patients as opposed to normals, presumably reflecting their activated state (717, 828-830). Interest has centered on potential effects on eosinophils; elevated GM-CSF, eotaxins and RANTES from ECs likely contributes to the recruitment and survival of these cells in CRSwNP. One study did demonstrate decreased IL-8 in cultures from CRSsNP patients suggesting that diminished neutrophil recruitment may play a role in pathogenesis (831). Recent in vivo studies demonstrated elevated EC expression of IL-32 in CRSsNP (832). In CRSwNP, elevated secretion of IL-6 (598) and BAFF (600) were observed, and this was at least in part from EC activity. BAFF (also called BLys or TNFSF13B) triggers B-cell proliferation and class switching, and these processes may have particular significance in CRS pathophysiology (600). BAFF is secreted by multiple cell types and will be discussed more extensively in the section on B cells.
EC cytokines have established effects on multiple cell types, including not only effector cells but also dendritic cells. Relevant to CRS, in vitro studies indicate that ECs from nasal polyps have the capacity to skew dendritic cell polarization in the Th2 direction (833). Mechanistically, it has been suggested that a subset of EC cytokines (IL-25, IL-33 and TSLP) are key determinants of dendritic cell polarization and subsequent T cell differentiation in response to mucosal antigens (601, 787, 788, 834). Specifically, these cytokines have the capacity to skew T cell differentiation in the Th2 direction, the pattern observed in Western CRSwNP patients. TSLP, in particular, has the ability to act directly on dendritic cells, shaping the T cell profile as well as directly and indirectly (through mast cells) recruiting eosinophils (601, 827). Whether TSLP is relevant to CRS pathogenesis is unclear, however a recent paper demonstrated elevated TSLP activity using a bioassay of supernatant from nasal polyp explants (835).
These results were independent of allergic status. Subsequent papers have also implicated TSLP in polyposis (836-838). Levels of other epithelial cytokines with Th2 properties, such as IL-33, have been reported as higher in recalcitrant CRSwNP (839) and genetic studies also suggest that variation near the IL-33 gene is associated with CRSwNP (840). In regard to IL-25, there is no current evidence for elevated expression or activity of this cytokine in CRS. Overall, crosstalk between ECs and dendritic cells remains an active area of CRS research.
4.2.4.2.1. Receptors
In addition to the physical barrier, sinonasal epithelial cells (ECs) play an active role in both the innate and acquired immune response (787, 788). Membrane bound and cytoplasmic pattern recognition receptors (PRRs) that recognize pathogen associated molecular patterns (PAMPs) have been identified on airway epithelial cells (139, 159, 789-791). PAMPs are conserved molecular patterns found in parasites, viruses, yeasts, bacteria and mycobacteria; recognition by host epithelial cells through PRRs results in the release of innate protective agents as well as chemokines and cytokines that attract innate cellular defenses (e.g. neutrophils). In addition to PAMPs, cells also sense cellular damage through damage-associated molecular patterns (DAMPs) (159, 792). The combined signal of foreign material plus cellular damage triggers an innate response and sets in motion, and ultimately helps determine the nature of, the adaptive immune response (793).
Prominent amongst the PRRs are the Toll-like receptors (TLRs), currently a family of 10 integral membrane glycoproteins that recognize extracellular or intracellular PAMPs such as bacterial cell-surface lipopolysaccharides (LPS) or unmethylated CpG islands found in pathogen DNA. Engagement of the TLRs by a PAMP triggers intracellular signaling through adapter proteins like MyD88 or TRIF that in turn can effect pro-inflammatory gene expression through the activation of nuclear transcription factors such as NF-kB, AP-1 and IRF3 (794). Given that TLR2, TLR3, TLR4 and TLR9 in particular are expressed on airway epithelium, it is likely they play an important role in mediating host inflammation, with potential derangements contributing to the development of CRS (795). This hypothesis is supported by the quantitative increase in TLR2 mRNA seen in cystic fibrosis polyps and in some studies of CRSsNP, (796, 797), as well as reported decreases in mucosal TLR2 and TLR9 mRNA in samples from CRSwNP (791, 798). These results have not been confirmed at the protein level nor is there data demonstrating a functional deficit of TLR signaling in CRS patients. Nevertheless, this remains a theoretical mechanism that can account for chronic mucosal inflammation and merits further exploration. Data regarding dysregulation of other PRRs in CRS is sparse, although NOD-like receptors (NLRs) are expressed in nasal and sinus epithelial cells (799). A single study indicated that levels were increased in CRSwNP epithelium and this level was decreased after nasal steroid use (800).
In addition to PRRs, sinonasal epithelial cells also express protease-activated receptors (PAR) (720). Although not classically considered host defense molecules, these receptors are activated by a variety of endogenous and exogenous proteases, including those associated with bacteria, fungi and allergens (801). Triggering of PAR receptors invokes the NFκβ signaling pathway that results in cytokine and chemokine production, cellular recruitment and potentially, skewing of the both the innate and acquired immune response (794, 802). In vitro studies on the nasal epithelium have indicated that PAR-2 activation triggers IL-8 release and this response can be elicited by Staphylococcus aureus-derived proteases (720, 803). Other investigators have suggested that fungal proteases may act on PAR receptors to drive both a neutrophilic and eosinophilic response (718). As mentioned earlier LEKT1 protein, a natural anti-protease, is reduced in CRSwNP epithelium (782). In addition to protecting tight junctions discussed above, this protein should also serve to shield epithelialbased PAR receptors. In a model of skin disease, a recent study demonstrated that the absence of LEKT1 leads to the expression of the Th2 skewing molecule TSLP via activation of PAR-2 (804). It has been suggested that LEKT deficiency may contribute to the pathogenesis of CRS via inappropriate PAR stimulation as well (597). This may be of particular significance given that CRSsNP and CRSwNP both express higher levels of PAR2 in comparison to normal ECs (720).
4.2.4.2.2. Epithelial Cell Response: Host defense molecules
Epithelial cells secrete a vast arsenal of antimicrobial molecules in several classes including enzymes (lysozyme, chitinases and peroxidases), opsonins (complement and pentraxin-3), permeabilizing proteins (A defensins, B defensins and cathelicidins such as LL-37), collectins (surfactant protein-A, surfactant protein-D and mannosebinding lectin) and binding proteins (lactoferrin and mucins) (611, 805, 806). Studies of CRS patients have not demonstrated a universal trend in the expression of these antimicrobial molecules. Levels for complement components, LL-37, surfactant protein A (SP-A) and Acid Mammalian Chitinase demonstrated increases, presumably compensatory, in CRS patients (735, 807-811). Lactoferrin and the S100 group of antimicrobials were decreased in CRS, however (686, 812, 813). The S100 proteins are products of a multi-gene family widely expressed in epithelial cells. In addition to direct anti-microbial effects, these have diverse effects on cell differentiation and wound healing, linking the mechanical barrier and classic anti-microbial properties (814). PLUNC (Palate Lung Nasal Epithelial Clone), another secreted antimicrobial protein, is decreased in CRSwNP (815). PLUNC is secreted by glandular rather than surface epithelium and this protein may have particular relevance for CRS as it possesses anti-biofilm properties.
Sinonasal epithelial cells (ECs) play an active role in both the innate and acquired immune response
Presence of diminished host defense molecules in CRS suggests the hypothesis that a primary sinonasal innate immune defect may contribute to local microbial proliferation and the development of CRS in a subset of patients (597). There is some evidence however that Th2 cytokines can cause nasal epithelial cells to down regulate the production of innate immune molecules such as human beta-defensin 2 and surfactant protein A (816). This suggests the alternative hypothesis that an inappropriate Th2 effector response at the mucosal surface may account for the observed innate immune deficiencies. Mechanistic studies to uncover whether diminished EC innate immune responses in CRS are constitutive and pre-exist the onset of CRS or are inducible responses to Th2 inflammation are still incomplete. Nevertheless, innate immune responses in ECs can be induced by the T cell cytokine IL-22, which works through its receptor IL-22R (817, 818). Binding of IL-22 to its receptor activates the transcription factor STAT 3, which mediates mucosal host defense and epithelial repair (819, 820). In airway ECs, the STAT 3 pathway regulates production of host defense molecules including the S100 family (821). Studies in the gut and lung indicate that this pathway is critical in the regulation of inflammatory responses at the epithelial surface in general (822). In CRSwNP, diminished expression of IL-22R has been reported (823) and separate studies have indicated that the STAT 3 pathway is blunted in CRSwNP (598). Interestingly, STAT 3 mutations have been indentified in Hyper IgE syndrome (HIES or Job's syndrome), which is associated with eosinophilia, high IgE, staph abscesses and recurrent sino-pulmonary infections (599). The similarities between CRSwNP and some aspects of Job's syndrome suggest the hypothesis that nasal polyposis may result from local (sinonasal) impairment of the STAT 3 pathway (598).
4.2.4.2.3. Epithelial Cell Response: Cytokines and Chemokines
Airway epithelial cells produce a variety of inflammatory cytokines, typically in response to PRR and PAR receptor stimulation (794). A partial list includes IL-1, TNF-α, IFNα/β, GM-CSF, eotaxins, RANTES, IP-10, IL-6, IL-8, GRO-α, MDC, SCF, TARC, MCP-4, BAFF, osteopontin, IL-25, IL-32, IL-33 and TSLP (24, 600, 805, 824-826). In addition to driving pain, swelling, vascular dilation and leak and other hallmarks of inflammation, many of these cytokines have chemokine properties attracting various leukocytes including eosinophils, mast cells, neutrophils, dendritic cells and lymphocytes. EC cytokines are also believed to play a key role in dendritic cell polarization, shaping the nature of the T cell response to antigens (827). Given the important role of ECs in mucosal immunity, altered nasal EC cytokine release may play a role in CRS pathogenesis. The contribution of EC gene expression to the overall mucosal cytokine milieu can be difficult to determine. Quantitative cytokine studies in CRS have been done on whole tissue biopsies for the most part, as the techniques needed to analyze isolated nasal EC secretion are more problematic. In vivo epithelial scrapings and in vitro EC cultures both have limitations: the former is generally limited to mRNA analysis as adequate protein samples are difficult to obtain while the latter is technically difficult and prone to potential cell culture effects. In vitro studies have most commonly demonstrated elevated EC cytokine secretion from CRS patients as opposed to normals, presumably reflecting their activated state (717, 828-830). Interest has centered on potential effects on eosinophils; elevated GM-CSF, eotaxins and RANTES from ECs likely contributes to the recruitment and survival of these cells in CRSwNP. One study did demonstrate decreased IL-8 in cultures from CRSsNP patients suggesting that diminished neutrophil recruitment may play a role in pathogenesis (831). Recent in vivo studies demonstrated elevated EC expression of IL-32 in CRSsNP (832). In CRSwNP, elevated secretion of IL-6 (598) and BAFF (600) were observed, and this was at least in part from EC activity. BAFF (also called BLys or TNFSF13B) triggers B-cell proliferation and class switching, and these processes may have particular significance in CRS pathophysiology (600). BAFF is secreted by multiple cell types and will be discussed more extensively in the section on B cells.
EC cytokines have established effects on multiple cell types, including not only effector cells but also dendritic cells. Relevant to CRS, in vitro studies indicate that ECs from nasal polyps have the capacity to skew dendritic cell polarization in the Th2 direction (833). Mechanistically, it has been suggested that a subset of EC cytokines (IL-25, IL-33 and TSLP) are key determinants of dendritic cell polarization and subsequent T cell differentiation in response to mucosal antigens (601, 787, 788, 834). Specifically, these cytokines have the capacity to skew T cell differentiation in the Th2 direction, the pattern observed in Western CRSwNP patients. TSLP, in particular, has the ability to act directly on dendritic cells, shaping the T cell profile as well as directly and indirectly (through mast cells) recruiting eosinophils (601, 827). Whether TSLP is relevant to CRS pathogenesis is unclear, however a recent paper demonstrated elevated TSLP activity using a bioassay of supernatant from nasal polyp explants (835).
These results were independent of allergic status. Subsequent papers have also implicated TSLP in polyposis (836-838). Levels of other epithelial cytokines with Th2 properties, such as IL-33, have been reported as higher in recalcitrant CRSwNP (839) and genetic studies also suggest that variation near the IL-33 gene is associated with CRSwNP (840). In regard to IL-25, there is no current evidence for elevated expression or activity of this cytokine in CRS. Overall, crosstalk between ECs and dendritic cells remains an active area of CRS research.
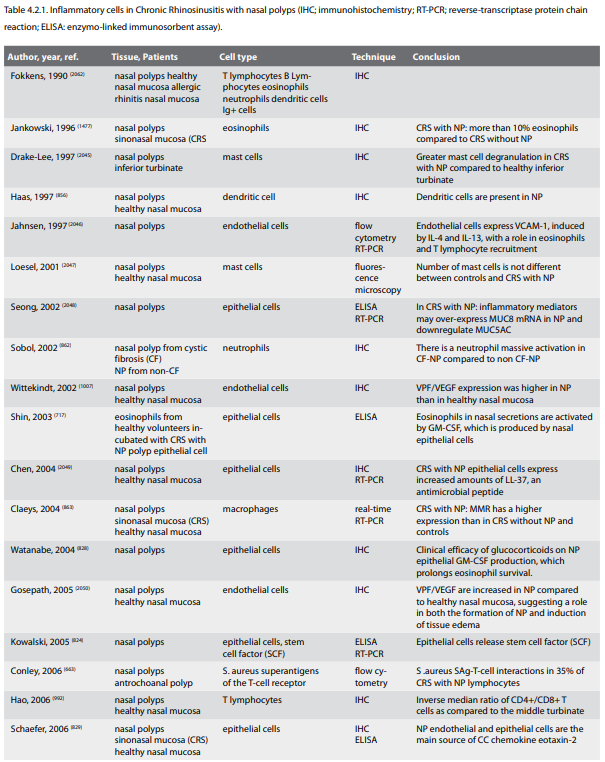
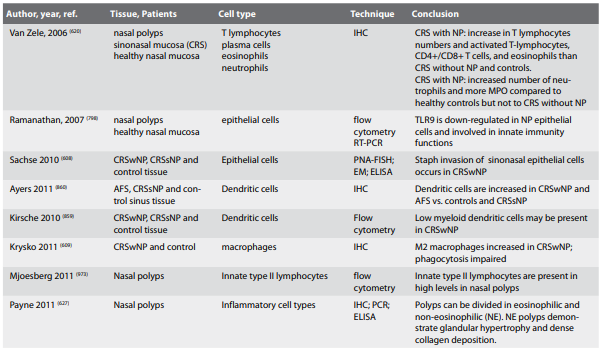
ECs likely play a significant role in mediating not only the
innate response, but also shaping the subsequent adaptive immune
response. Whether primary variations in ECs responses underlie
CRS aetiology and pathogenesis is unclear but interfering with
EC cytokine expression for therapeutic purposes is an area of
active research (841, 842). Furthermore, in contrast to the
up-regulation of antimicrobials in EC, corticosteroids down
regulate EC cytokine secretion (828, 843, 844). This bimodal
effect of corticosteroids on ECs may be a key mechanism
accounting for their efficacy in CRS.
4.2.4.2.4. Epithelial Cell Response: Co-stimulatory molecules
In addition to cytokine mediated regulation of T-cells mentioned above, airway epithelial cells also express homologues of the B7 co-stimulatory family (192). Expression of these cell surface ligands, which have the ability to down regulate T-cell responses, are increased in CRS patients and induced by TNF-α and IFN-γ (191). Induction of B7 molecules also occurs via viral infection (190). The clinical significance of this down regulation of T-cells and its possible relevance to viral exacerbations of CRS remains unclear.
4.2.4.2.5. Epithelial Cell Response: Inflammatory Enzymes, ROS and RNS
Enzymes involved in the generation of reactive oxygen species (ROS) are important in multiple epithelial processes including mucin production, epithelial repair, innate immunity and response to environmental toxins (805). Oxidative enzymes are important in the generation of hypothiocyanite, an important antimicrobial that selectively kills microorganisms and spares host cells; this pathway is defective in cystic fibrosis (805). Environmental toxins induce ROS production, which is counteracted by various scavenger enzymes and anti-oxidants in airway epithelial cells. If these protective mechanisms are overwhelmed, pro-inflammatory cytokines are induced; additional oxidative stress can lead to cell death (845).
Reactive nitrogen species (RNS) also play a significant role in several biologic processes and RNS can also interact with ROS in disease causing tissue damage (759). In particular, there has been a great deal of interest in a potential role for nitric oxide in CRS. Nitric oxide (NO) is produced by nitric oxide synthase (NOS) and there are 3 relevant enzymes in the airway: inducible NOS (iNOS), and endothelial and neural NOS (eNOS and nNOS), which are constitutive. A variety of cells types possess iNOS, including epithelial cells and macrophages. Stimuli for induction of iNOS include various chemokines, cytokines, allergens, viruses, pollutants, hypoxia, bacterial toxins and viruses (805). In general, constitutive NO acts as an intracellular messenger and neurotransmitter, induced NO mediates inflammatory and antimicrobial effects and can also regulate apoptosis. The sinuses produce very high amounts of NO and it has been proposed that this limits bacterial colonization of these structures given the proximity of the nasal and oral cavity (846). NO also regulates ciliary beat frequency (847) and studies have indicated low levels of nasal NO in CRSsNP (848). The lowest levels of NO have been reported in CRSwNP, and levels increase with treatment (849, 850). These reports have generated a large number of studies on the topic of nitric oxide and CRS, but the role of nasal NO in health and disease has not been clarified, in part due to variations in methodology. No study to date has correlated nNO levels with any clinical, molecular or pathological measure of sinus mucosal inflammation (851). In terms of aetiology and pathogenesis of CRS, it has been suggested that metabolic pathways are abnormal in nasal polyposis (852) and that high NO levels are important in keeping microbial colonization levels low within the paranasal sinuses (853). In particular, high levels of NO have inhibitory effects of S. aureus biofilm growth (854) but these levels may actually promote growth of other bacteria. The clinical relevance also remains unclear since ESS, which has a high success rate in most studies, reduces the NO concentration in operated sinuses (855). Moreover, a fundamental criticism of existing work on the topic is that all studies measure NO in the sinus lumen, rather than at the mucosal surface and in the respiratory mucus where innate defenses operate (851).
4.2.4.3. Dendritic Cells and Macrophages
Dendritic cells (DCs) activate both innate and adaptive immunity via antigen capture, presentation of antigen to immature T cells and secretion of soluble inflammatory mediators. Crosstalk between epithelial cells and DCs (see discussion above) is believed crucial to the determination of any subsequent T cell response to mucosal antigen and these cells serve as a bridge between the innate and adaptive response (601). DCs have been described in the nasal mucosa (856) and a recent study indicates that multiple subsets are present (857). Studies in CRS have been limited but functional DCs are present in polyp mucosa (858). It has been suggested that myeloid dendritic cells are decreased in the polyps when compared to CRSsNP or control nasal tissues and this accounts for the observed Th2 skewing (859). Other investigators demonstrated increased DCs in CRSwNP vs. either CRSsNP or control mucosa (860). In this study, elevated DC chemoattractants CCL2 and CCL20 were present in polyp mucosa suggesting recruitment of immature DCs to the sinonasal mucosa. DCs were increased in CRSsNP vs. control mucosa but the difference was not significant. Levels of Vitamin D3, an immunoregulatory molecule with known effects on DCs, were low in CRSwNP suggesting a potential role for replacement therapy (833). More broadly, the key role of DCs in the mucosal immune response makes them attractive targets for the management of chronic airway inflammation; in particular, modulating epithelial/DC crosstalk may have therapeutic value (601). Macrophages are innate immune cells with diverse roles: removal of particulates, primary response to pathogens; tissue homeostasis; coordination of the adaptive immune response; inflammation; tissue repair (861). The classical macrophage activation pathway (M1) is driven by Th1 cytokines that trigger a pro-inflammatory response necessary to kill intracellular pathogens. The alternative pathway is driven by Th2 cytokines in the local milieu leading to M2 macrophages; this process is important in the defense against helminthes, humoral immunity and tissue repair (861). Macrophages (presumably mostly M1) are elevated in the sinonasal mucosa of cystic fibrosis (CF) patients in comparison to controls and CRS (862). M2 macrophages, which express elevated levels of the macrophage mannose receptor (MMR), are present in high levels of CRSwNP patients as opposed to CRSsNP, CF and controls (609, 797, 863). Eosinophils, via CCL23, may be key to the recruitment of macrophages in CRSwNP, which then convert to the M2 type in the Th2 milieu (610). These polyp-derived macrophages appear to have an impaired ability to phagocytose S. aureus, which may contribute to the pathophysiology of CRSwNP (609). In addition, M2 macrophages derived from nasal polyps secrete high levels of CCL18, a cytokine known to be chemotactic for DCs, naïve t cells and Th2 cells all of which may contribute to the pathogenesis of CRSwNP (832).
4.2.4.4. Eosinophils
Eosinophils are circulating granulocytes whose function at mucosal surfaces is immune defense, primarily against multi-cellular parasites. In addition, it has been suggested that eosinophils play a significant role in tissue remodeling and repair in both health and disease (864). Their presence in high numbers in the respiratory mucosa however, has long been associated with disease, most prominently asthma and allergic rhinitis. Eosinophils are also an important cell type in chronic rhinosinusitis, and CRS was at one time considered by many to be a purely eosinophilic disease. Eosinophilic damage to the sinonasal mucosa was believed to be the central pathophysiologic mechanism of CRS and the hallmark of the disorder (38, 865). Significantly, the degree of eosinophilia in CRS was independent of the concomitant presence of allergic rhinitis, suggesting distinct but possibly overlapping pathophysiologic processes (744, 866). In addition, the degree of tissue eosinophilia in CRS correlates with objective disease severity and co-morbid asthma (542, 867-870). The introduction of the 'fungal hypothesis' (see section on fungi) further enhanced the role of the eosinophil; toxic mediators released by eosinophils targeting fungi were proposed as the common upstream pathway for all forms of CRS (592, 699). Variation in the degree of tissue eosinophilia in surgical specimens was believed to reflect the presence or absence of allergic rhinitis, prior corticosteroid use or simply disease-intensity. It was always clear however, that non-eosinophilic forms of nasal polyposis existed, most obviously in cases of cystic fibrosis (871) but this was considered an exception. The concept of tissue eosinophilia is relative however, and some cases of CRS demonstrated relatively minimal eosinophilia and the predominant influx of other cell types. Notably, separation of CRS tissue specimens into CRSsNP and CRSwNP demonstrated that tissue eosinophilia was much higher in the polypoid form (620, 866, 872-874). This close association, independent of atopy, suggested that eosinophils may be critical to polyp formation but the relationship between CRSwNP and mucosal eosinophilia is not maintained in Asian polyps (875) as well as a demonstrable minority of Western/ Caucasian polyps (626). While approximately 80% of Caucasian polyps are eosinophilic, less than 50% of Asian polyps demonstrate tissue eosinophilia above that seen in control tissues (875-877). In addition, the majority of CRSsNP worldwide appears to be relatively non-eosinophilic, at least in comparison to Caucasian polyps. Taken together, these studies indicate that eosinophils are not absolutely necessary for nasal polyposis or CRS to be present. Although this might appear to diminish the importance of these cells in CRS, a recent longitudinal study demonstrated that high tissue eosinophilia correlated directly with the need for revision surgery (878). A second well done prospective study divided patients by polyp status and tissue eosinophilia. Results indicated that CRSsNP patients with high tissue eosinophilia, while less common, nevertheless demonstrated the least improvement of the four groups with surgical therapy (879). Consequently, while eosinophils are not essential for CRS to exist, they appear to be a biomarker for severe, recalcitrant disease, at least in Caucasians, and may still be the cell that mediates this relatively poor prognosis (880).
Eosinophil levels and Th2 cytokine skewing are most closely associated with Western CRSwNP.
The mechanism of recruitment and activation of eosinophils in CRS involves 3 main processes:
1 the local expression of eosinophil-attracting chemokines by the epithelium and other cell types
2 priming and survival promoting effects of cytokines such as GM-CSF and IL-5 and
3 the expression of adhesion molecules by endothelium especially VCAM-1.
The relevant chemokines are RANTES, Eotaxin 1-3, MCP 1-4, all primarily secreted by nasal epithelial cells and all of which work through CCR3 (841, 881-891). In allergic inflammation, other cellular sources, such as dendritic cells and macrophages, may be the most important sources of eotaxin and other CCR3 ligands.
The regulation of epithelial chemokine expression is complex, but the Th2 cytokines IL-4 and IL-13 play a key role working through STAT6 and NF-κβ (892, 893). Other stimuli such as chitin (see above) may play a role as well (739). In addition, eosinophils secrete eotaxin 1-3 as well as RANTES, suggesting a possible amplifying effect enhancing local eosinophil recruitment (891, 894). The relevant cytokines GM-CSF and IL-5 induce increased migration, adhesion and survival of eosinophils in nasal polyp tissue. GM-CSF was identified first, and is produced in particular, by epithelial cells (524, 895-899). IL-5 is also an important priming and survival factor for eosinophils in nasal polyps (900-903). Initially, IL-5 levels in nasal polyp tissue were believed to correlate with atopic status (524) but multiple follow up studies indicated that IL-5 status-and hence any effect on eosinophils-was independent of systemic allergy (542, 900, 904, 905). The most relevant adhesion molecule appears to be VCAM-1, which mediates rolling, adhesion and transendothelial migration of eosinophils in vitro. Several groups have demonstrated increased expression in nasal polyps and levels correlate with the presence of eosinophils (883, 906-910). A recent study indicated high VCAM1 levels correlated with risk of post surgical recurrence (910). P-selectin is an additional adhesion molecule that may also play a role in eosinophil accumulation within nasal polyps (911) while L-selectin appears to regulate eosinophil accumulation in CRSsNP (18, 912).
Asian polyps are less eosinophilic than Western CRSwNP, exhibiting a Th1/17 cytokine skewing
The overall process of eosinophil recruitment, activation and survival in CRS, when present, is likely driven primarily by Th2 cytokines via the mechanisms discussed above. The critical upstream cellular sources of these Th2 cytokines in eosinophilic CRS remain unclear, but presumably include Th2 helper T-cells. In CRSwNP, substantial evidence exists that staphylococcal superantigens promote mucosal eosinophilia primarily by accentuating local Th2 cytokine release via actions on these T-cells, although other mechanisms may also be relevant (542, 621). Very recent evidence has further suggested that staphylococcal biofilms may play an additional role driving eosinophilia in CRS, independent of polyp status or superantigens (603). The mechanism for this potential effect is uncertain and further studies will be necessary to validate this hypothesis. As mentioned earlier, based primarily on in vitro data, the 'fungal hypothesis' proposed that Alternaria fostered tissue eosinophilia via accentuation of Th2 cytokine release from sensitized T-cells (593, 702). Two follow up studies failed to confirm these in vitro observations however (706, 707) and the weight of evidence does not support a major role for fungi in most forms of CRS at this time (25, 697, 713, 913). Other factors including IL-33, TSLP, IL-25, PAR receptors, complement proteins, eicosanoids and Stem Cell Factor may play an upstream role in CRS tissue eosinophilia but evidence is currently very limited (601, 718, 809, 824, 835, 840, 914, 915). Once present and activated, eosinophils are believed to damage the mucosa through degranulation and release of toxic mediators with resulting epithelial sloughing and tissue oedema (865, 916, 917). In addition to direct toxic effects, eosinophils in nasal polyps express CCL23, which acts to recruit macrophages and monocytes, whose products may also contribute to the inflammation in CRSwNP (610).The mechanism for eosinophil de-granulation in CRS is unclear but data from other tissues suggests that crosslinking of receptors for IgA is an important trigger (918, 919). Effects on eosinophils by IgA can occur even in the absence of antigen binding (920). High levels of IgA have been identified in nasal polyps suggesting that this immunoglobulin may play a key role in vivo (621, 921). Lastly, it has been proposed that the epithelial barrier in CRS is already weakened (25, 782), thus eosinophilic degranulation should only accentuate the process. In addition to the above noted pathologic effects, eosinophils in lower airway disease foster fibrotic changes of the sub epithelial tissues with the laying down of extracellular proteins (922, 923). Eosinophil production of PDGF as well as TGFα and β-1 may alter the structure of affected nasal mucosa (924-926). Ultrastructural studies on nasal polyps treated with anti-IL-5 will be required to more definitively address the role of eosinophils in the remodeling of CRS sinonasal tissue (see below). The association of eosinophilia with refractory disease makes this cell a potentially important target in CRS. Eosinophils are steroid-responsive (927) and this likely explains at least some of the therapeutic effects of glucocorticoids in CRS (27). A large body of literature indicates that glucocorticoids can inhibit eosinophil recruitment, survival and activation in CRS (880). A recent doubleblind trial using oral corticosteroids demonstrated clinical efficacy as well as reduced IL-5 and ECP in nasal secretions (928). Targeted therapy using anti-IL-5 in CRSwNP has shown promise as well. IL-5 and its receptor are both elevated in Caucasian (eosinophilic) nasal polyps (929, 930). Clinical trials using anti-IL-5 antibodies demonstrated evidence for reduced polyp eosinophilia as well as clinical efficacy (931, 932).
4.2.4.2.4. Epithelial Cell Response: Co-stimulatory molecules
In addition to cytokine mediated regulation of T-cells mentioned above, airway epithelial cells also express homologues of the B7 co-stimulatory family (192). Expression of these cell surface ligands, which have the ability to down regulate T-cell responses, are increased in CRS patients and induced by TNF-α and IFN-γ (191). Induction of B7 molecules also occurs via viral infection (190). The clinical significance of this down regulation of T-cells and its possible relevance to viral exacerbations of CRS remains unclear.
4.2.4.2.5. Epithelial Cell Response: Inflammatory Enzymes, ROS and RNS
Enzymes involved in the generation of reactive oxygen species (ROS) are important in multiple epithelial processes including mucin production, epithelial repair, innate immunity and response to environmental toxins (805). Oxidative enzymes are important in the generation of hypothiocyanite, an important antimicrobial that selectively kills microorganisms and spares host cells; this pathway is defective in cystic fibrosis (805). Environmental toxins induce ROS production, which is counteracted by various scavenger enzymes and anti-oxidants in airway epithelial cells. If these protective mechanisms are overwhelmed, pro-inflammatory cytokines are induced; additional oxidative stress can lead to cell death (845).
Reactive nitrogen species (RNS) also play a significant role in several biologic processes and RNS can also interact with ROS in disease causing tissue damage (759). In particular, there has been a great deal of interest in a potential role for nitric oxide in CRS. Nitric oxide (NO) is produced by nitric oxide synthase (NOS) and there are 3 relevant enzymes in the airway: inducible NOS (iNOS), and endothelial and neural NOS (eNOS and nNOS), which are constitutive. A variety of cells types possess iNOS, including epithelial cells and macrophages. Stimuli for induction of iNOS include various chemokines, cytokines, allergens, viruses, pollutants, hypoxia, bacterial toxins and viruses (805). In general, constitutive NO acts as an intracellular messenger and neurotransmitter, induced NO mediates inflammatory and antimicrobial effects and can also regulate apoptosis. The sinuses produce very high amounts of NO and it has been proposed that this limits bacterial colonization of these structures given the proximity of the nasal and oral cavity (846). NO also regulates ciliary beat frequency (847) and studies have indicated low levels of nasal NO in CRSsNP (848). The lowest levels of NO have been reported in CRSwNP, and levels increase with treatment (849, 850). These reports have generated a large number of studies on the topic of nitric oxide and CRS, but the role of nasal NO in health and disease has not been clarified, in part due to variations in methodology. No study to date has correlated nNO levels with any clinical, molecular or pathological measure of sinus mucosal inflammation (851). In terms of aetiology and pathogenesis of CRS, it has been suggested that metabolic pathways are abnormal in nasal polyposis (852) and that high NO levels are important in keeping microbial colonization levels low within the paranasal sinuses (853). In particular, high levels of NO have inhibitory effects of S. aureus biofilm growth (854) but these levels may actually promote growth of other bacteria. The clinical relevance also remains unclear since ESS, which has a high success rate in most studies, reduces the NO concentration in operated sinuses (855). Moreover, a fundamental criticism of existing work on the topic is that all studies measure NO in the sinus lumen, rather than at the mucosal surface and in the respiratory mucus where innate defenses operate (851).
4.2.4.3. Dendritic Cells and Macrophages
Dendritic cells (DCs) activate both innate and adaptive immunity via antigen capture, presentation of antigen to immature T cells and secretion of soluble inflammatory mediators. Crosstalk between epithelial cells and DCs (see discussion above) is believed crucial to the determination of any subsequent T cell response to mucosal antigen and these cells serve as a bridge between the innate and adaptive response (601). DCs have been described in the nasal mucosa (856) and a recent study indicates that multiple subsets are present (857). Studies in CRS have been limited but functional DCs are present in polyp mucosa (858). It has been suggested that myeloid dendritic cells are decreased in the polyps when compared to CRSsNP or control nasal tissues and this accounts for the observed Th2 skewing (859). Other investigators demonstrated increased DCs in CRSwNP vs. either CRSsNP or control mucosa (860). In this study, elevated DC chemoattractants CCL2 and CCL20 were present in polyp mucosa suggesting recruitment of immature DCs to the sinonasal mucosa. DCs were increased in CRSsNP vs. control mucosa but the difference was not significant. Levels of Vitamin D3, an immunoregulatory molecule with known effects on DCs, were low in CRSwNP suggesting a potential role for replacement therapy (833). More broadly, the key role of DCs in the mucosal immune response makes them attractive targets for the management of chronic airway inflammation; in particular, modulating epithelial/DC crosstalk may have therapeutic value (601). Macrophages are innate immune cells with diverse roles: removal of particulates, primary response to pathogens; tissue homeostasis; coordination of the adaptive immune response; inflammation; tissue repair (861). The classical macrophage activation pathway (M1) is driven by Th1 cytokines that trigger a pro-inflammatory response necessary to kill intracellular pathogens. The alternative pathway is driven by Th2 cytokines in the local milieu leading to M2 macrophages; this process is important in the defense against helminthes, humoral immunity and tissue repair (861). Macrophages (presumably mostly M1) are elevated in the sinonasal mucosa of cystic fibrosis (CF) patients in comparison to controls and CRS (862). M2 macrophages, which express elevated levels of the macrophage mannose receptor (MMR), are present in high levels of CRSwNP patients as opposed to CRSsNP, CF and controls (609, 797, 863). Eosinophils, via CCL23, may be key to the recruitment of macrophages in CRSwNP, which then convert to the M2 type in the Th2 milieu (610). These polyp-derived macrophages appear to have an impaired ability to phagocytose S. aureus, which may contribute to the pathophysiology of CRSwNP (609). In addition, M2 macrophages derived from nasal polyps secrete high levels of CCL18, a cytokine known to be chemotactic for DCs, naïve t cells and Th2 cells all of which may contribute to the pathogenesis of CRSwNP (832).
4.2.4.4. Eosinophils
Eosinophils are circulating granulocytes whose function at mucosal surfaces is immune defense, primarily against multi-cellular parasites. In addition, it has been suggested that eosinophils play a significant role in tissue remodeling and repair in both health and disease (864). Their presence in high numbers in the respiratory mucosa however, has long been associated with disease, most prominently asthma and allergic rhinitis. Eosinophils are also an important cell type in chronic rhinosinusitis, and CRS was at one time considered by many to be a purely eosinophilic disease. Eosinophilic damage to the sinonasal mucosa was believed to be the central pathophysiologic mechanism of CRS and the hallmark of the disorder (38, 865). Significantly, the degree of eosinophilia in CRS was independent of the concomitant presence of allergic rhinitis, suggesting distinct but possibly overlapping pathophysiologic processes (744, 866). In addition, the degree of tissue eosinophilia in CRS correlates with objective disease severity and co-morbid asthma (542, 867-870). The introduction of the 'fungal hypothesis' (see section on fungi) further enhanced the role of the eosinophil; toxic mediators released by eosinophils targeting fungi were proposed as the common upstream pathway for all forms of CRS (592, 699). Variation in the degree of tissue eosinophilia in surgical specimens was believed to reflect the presence or absence of allergic rhinitis, prior corticosteroid use or simply disease-intensity. It was always clear however, that non-eosinophilic forms of nasal polyposis existed, most obviously in cases of cystic fibrosis (871) but this was considered an exception. The concept of tissue eosinophilia is relative however, and some cases of CRS demonstrated relatively minimal eosinophilia and the predominant influx of other cell types. Notably, separation of CRS tissue specimens into CRSsNP and CRSwNP demonstrated that tissue eosinophilia was much higher in the polypoid form (620, 866, 872-874). This close association, independent of atopy, suggested that eosinophils may be critical to polyp formation but the relationship between CRSwNP and mucosal eosinophilia is not maintained in Asian polyps (875) as well as a demonstrable minority of Western/ Caucasian polyps (626). While approximately 80% of Caucasian polyps are eosinophilic, less than 50% of Asian polyps demonstrate tissue eosinophilia above that seen in control tissues (875-877). In addition, the majority of CRSsNP worldwide appears to be relatively non-eosinophilic, at least in comparison to Caucasian polyps. Taken together, these studies indicate that eosinophils are not absolutely necessary for nasal polyposis or CRS to be present. Although this might appear to diminish the importance of these cells in CRS, a recent longitudinal study demonstrated that high tissue eosinophilia correlated directly with the need for revision surgery (878). A second well done prospective study divided patients by polyp status and tissue eosinophilia. Results indicated that CRSsNP patients with high tissue eosinophilia, while less common, nevertheless demonstrated the least improvement of the four groups with surgical therapy (879). Consequently, while eosinophils are not essential for CRS to exist, they appear to be a biomarker for severe, recalcitrant disease, at least in Caucasians, and may still be the cell that mediates this relatively poor prognosis (880).
Eosinophil levels and Th2 cytokine skewing are most closely associated with Western CRSwNP.
The mechanism of recruitment and activation of eosinophils in CRS involves 3 main processes:
1 the local expression of eosinophil-attracting chemokines by the epithelium and other cell types
2 priming and survival promoting effects of cytokines such as GM-CSF and IL-5 and
3 the expression of adhesion molecules by endothelium especially VCAM-1.
The relevant chemokines are RANTES, Eotaxin 1-3, MCP 1-4, all primarily secreted by nasal epithelial cells and all of which work through CCR3 (841, 881-891). In allergic inflammation, other cellular sources, such as dendritic cells and macrophages, may be the most important sources of eotaxin and other CCR3 ligands.
The regulation of epithelial chemokine expression is complex, but the Th2 cytokines IL-4 and IL-13 play a key role working through STAT6 and NF-κβ (892, 893). Other stimuli such as chitin (see above) may play a role as well (739). In addition, eosinophils secrete eotaxin 1-3 as well as RANTES, suggesting a possible amplifying effect enhancing local eosinophil recruitment (891, 894). The relevant cytokines GM-CSF and IL-5 induce increased migration, adhesion and survival of eosinophils in nasal polyp tissue. GM-CSF was identified first, and is produced in particular, by epithelial cells (524, 895-899). IL-5 is also an important priming and survival factor for eosinophils in nasal polyps (900-903). Initially, IL-5 levels in nasal polyp tissue were believed to correlate with atopic status (524) but multiple follow up studies indicated that IL-5 status-and hence any effect on eosinophils-was independent of systemic allergy (542, 900, 904, 905). The most relevant adhesion molecule appears to be VCAM-1, which mediates rolling, adhesion and transendothelial migration of eosinophils in vitro. Several groups have demonstrated increased expression in nasal polyps and levels correlate with the presence of eosinophils (883, 906-910). A recent study indicated high VCAM1 levels correlated with risk of post surgical recurrence (910). P-selectin is an additional adhesion molecule that may also play a role in eosinophil accumulation within nasal polyps (911) while L-selectin appears to regulate eosinophil accumulation in CRSsNP (18, 912).
Asian polyps are less eosinophilic than Western CRSwNP, exhibiting a Th1/17 cytokine skewing
The overall process of eosinophil recruitment, activation and survival in CRS, when present, is likely driven primarily by Th2 cytokines via the mechanisms discussed above. The critical upstream cellular sources of these Th2 cytokines in eosinophilic CRS remain unclear, but presumably include Th2 helper T-cells. In CRSwNP, substantial evidence exists that staphylococcal superantigens promote mucosal eosinophilia primarily by accentuating local Th2 cytokine release via actions on these T-cells, although other mechanisms may also be relevant (542, 621). Very recent evidence has further suggested that staphylococcal biofilms may play an additional role driving eosinophilia in CRS, independent of polyp status or superantigens (603). The mechanism for this potential effect is uncertain and further studies will be necessary to validate this hypothesis. As mentioned earlier, based primarily on in vitro data, the 'fungal hypothesis' proposed that Alternaria fostered tissue eosinophilia via accentuation of Th2 cytokine release from sensitized T-cells (593, 702). Two follow up studies failed to confirm these in vitro observations however (706, 707) and the weight of evidence does not support a major role for fungi in most forms of CRS at this time (25, 697, 713, 913). Other factors including IL-33, TSLP, IL-25, PAR receptors, complement proteins, eicosanoids and Stem Cell Factor may play an upstream role in CRS tissue eosinophilia but evidence is currently very limited (601, 718, 809, 824, 835, 840, 914, 915). Once present and activated, eosinophils are believed to damage the mucosa through degranulation and release of toxic mediators with resulting epithelial sloughing and tissue oedema (865, 916, 917). In addition to direct toxic effects, eosinophils in nasal polyps express CCL23, which acts to recruit macrophages and monocytes, whose products may also contribute to the inflammation in CRSwNP (610).The mechanism for eosinophil de-granulation in CRS is unclear but data from other tissues suggests that crosslinking of receptors for IgA is an important trigger (918, 919). Effects on eosinophils by IgA can occur even in the absence of antigen binding (920). High levels of IgA have been identified in nasal polyps suggesting that this immunoglobulin may play a key role in vivo (621, 921). Lastly, it has been proposed that the epithelial barrier in CRS is already weakened (25, 782), thus eosinophilic degranulation should only accentuate the process. In addition to the above noted pathologic effects, eosinophils in lower airway disease foster fibrotic changes of the sub epithelial tissues with the laying down of extracellular proteins (922, 923). Eosinophil production of PDGF as well as TGFα and β-1 may alter the structure of affected nasal mucosa (924-926). Ultrastructural studies on nasal polyps treated with anti-IL-5 will be required to more definitively address the role of eosinophils in the remodeling of CRS sinonasal tissue (see below). The association of eosinophilia with refractory disease makes this cell a potentially important target in CRS. Eosinophils are steroid-responsive (927) and this likely explains at least some of the therapeutic effects of glucocorticoids in CRS (27). A large body of literature indicates that glucocorticoids can inhibit eosinophil recruitment, survival and activation in CRS (880). A recent doubleblind trial using oral corticosteroids demonstrated clinical efficacy as well as reduced IL-5 and ECP in nasal secretions (928). Targeted therapy using anti-IL-5 in CRSwNP has shown promise as well. IL-5 and its receptor are both elevated in Caucasian (eosinophilic) nasal polyps (929, 930). Clinical trials using anti-IL-5 antibodies demonstrated evidence for reduced polyp eosinophilia as well as clinical efficacy (931, 932).
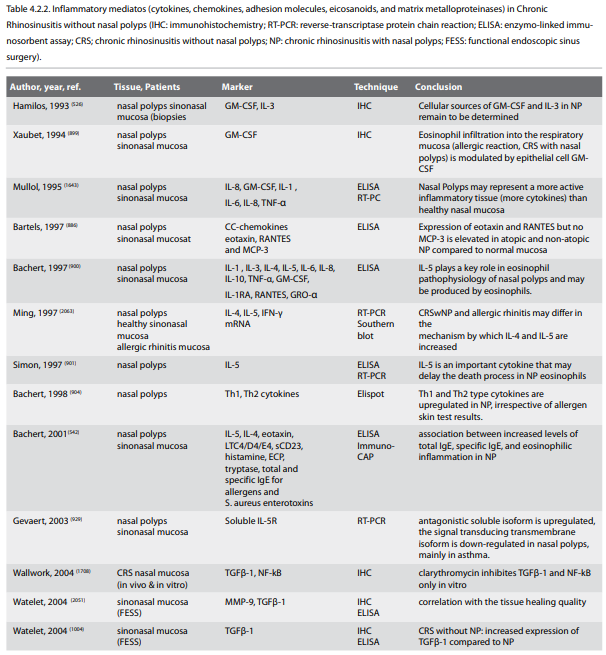
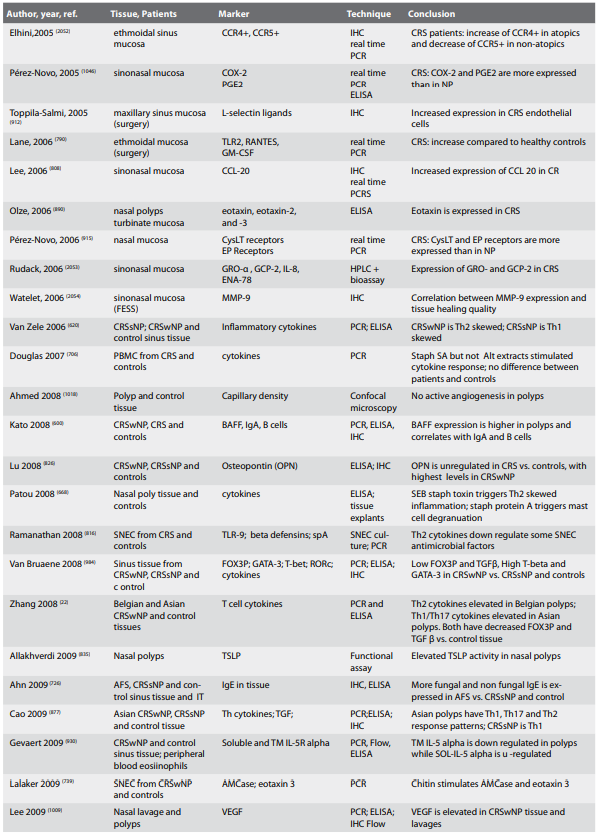
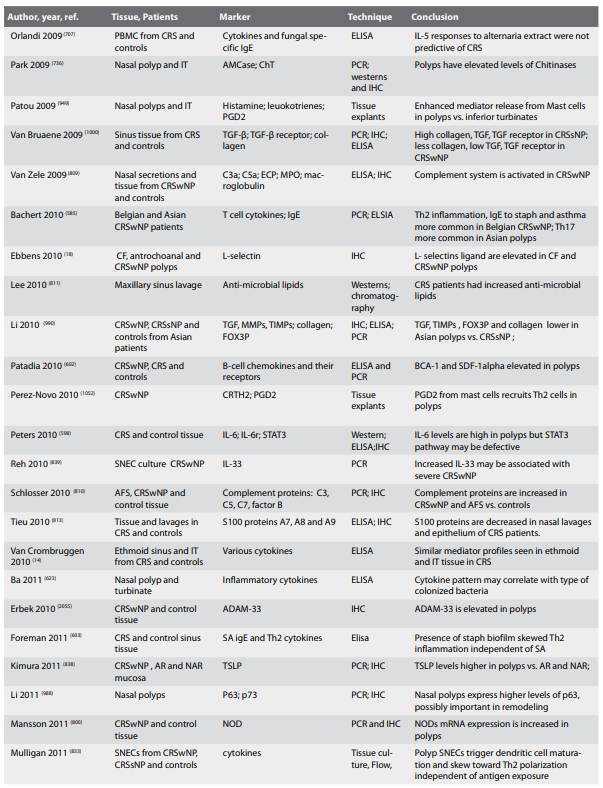
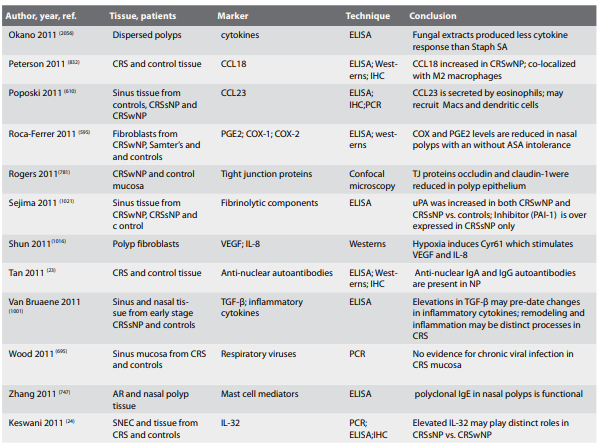
4.2.4.5. Neutrophils
Neutrophils are circulating immune effector cells with an established role in the early phagocytosis and killing of extracellular microbes. Recruitment to mucosal sites is typically driven by microbial stimulation of PRRs, with release of cytokines that trigger endothelial expression of selectins, integrin ligands and chemokines. The main chemokine fostering neutrophil recruitment in CRS appears to be IL-8, in part released by nasal epithelial cells in response to PAR2 stimulation (720). The role of the neutrophil in CRS remains unclear but the highest sinus tissue levels are seen in CF patients (862). For other forms of CRS, differences appear to depend on ethnicity as well as the presence or absence of nasal polyps. In Caucasians, neutrophilic infiltration can be demonstrated in CRS, with slightly lower levels observed in CRSsNP than in CRSwNP (620, 873, 874). In concert, studies have shown upregulation of IL-8 in both CRSwNP and CRSsNP (620, 933-935). Neutrophils did not appear to replace eosinophils in CRS mucosa, rather they were superimposed on the process; hence the term 'neutrophilic' rhinosinusitis was not considered completely appropriate for CRSsNP (874). Nevertheless, the degree of neutrophilic infiltrate was comparable between CRSsNP and CRSwNP as opposed to the eosinophilic infiltrate, which was significantly less in CRSsNP. As a corollary, it has been suggested that CRSsNP is more distinctly a neutrophilic process, while CRSwNP is more eosinophilic based on the relative degree of tissue infiltration (936). Furthermore, in the subpopulation of CRSwNP patients with relatively low eosinophilic infiltration, it has been suggested that neutrophils may be the major pathologic driver of disease, analogous to 'neutrophilic' asthma (19). In studies of polyps from Chinese patients, neutrophilic and eosinophilic infiltration appeared to be less than that seen in Caucasian polyps but the degree of eosinophilia was much more reduced, hence these polyps were relatively neutrophilic (22, 875). A later study on Chinese patients from a different region indicated that Asian CRSsNP patients were comparably much more neutrophilic than Asian CRSwNP patients (877). In the subset of Asian polyps that were non-eosinophilic however, significant neutrophilia was observed suggesting distinct underlying pathogenic processes within the CRSwNP group (877). Overall, it should be kept in mind that Asian polyps may be quite different in cellular and cytokine profile throughout the continent. Traditionally, neutrophils have been considered an acute response cell with a relatively short tissue half-life, therefore reasons for their accumulation in CRS are not completely clear. Recent studies have however, expanded the role of neutrophils beyond phagocytocis of extracellular organisms based in part on their diverse repertoire of effector molecules, which they express upon appropriate stimulation. In particular, neutrophils, may play a significant role in the resolution of inflammation as well as the pathology of the chronic inflammatory state (937). Chronic neutrophilic inflammation is observed in lung disorders such as COPD and CF, mediating extensive tissue injury and contributing to organ dysfunction. Neutrophil products include various proteolytic enzymes, which may alter the proteaseantiprotease balance triggering damage and remodeling. The excessive accumulation of neutrophils may be driven by the products derived from the breakdown of extracellular matrix, namely N-acetyl Pro-Gly-Pro (PGP) (938). PGP is normally metabolized but the process is impeded by cigarette smoke, with resulting inappropriate neutrophil accumulation in COPD (939). In CF lungs, low extracellular chloride levels, driven by the CFTR defect, has been proposed to diminish physiologic PGP breakdown (939). Whether these processes take place in CF polyps or neutrophilic CRS in general is unknown. Interestingly, this pathway links smoking with neutrophilic inflammation, a process suggested by a separate line of research in CRS (765). Nevertheless, they suggest a significant potential role for neutrophils in the pathophysiology of CRS and further suggest a molecular hypothesis for the negative effect of tobacco smoke on treatment outcomes.
4.2.4.6. Mast Cells
Mast cells are resident cells of the sinonasal mucosa with physiologic roles in innate immunity and wound healing (940). Activation of mast cells results in the release of pre-formed granules including histamine, serotonin, proteoglycans and serine proteases; in addition, de novo synthesis and secretion of various eicosanoids, chemokines and cytokines also takes place. Physiologic activation of mast cells in immune defense works in part through PPR stimulation (940). In nasal disease states, mast cell de-granulation has been most commonly implicated in allergic rhinitis via antigen-driven IgE cross-linking. In CRS, most interest has centered on a role for mast cells in nasal polyposis, in part due to the potential to induce, augment and maintain eosinophilic inflammation through IgE-dependent and IgE-independent processes (941, 942). In particular, polyp explant studies have demonstrated that mast cell de-granulation may be triggered directly by protein A (SpA), a staphylococcal surface protein (668). Mast cell prostaglandins have been implicated in Th2 lymphocyte recruitment and activation in nasal polyps (669). These results suggest that mast cells can activate Th2 lymphocytes independently of T-cell receptor activation, with attendant secretion of Th2 cytokines (943). Stem cell factor, secreted by epithelial cells, may be important in the recruitment of mast cells in nasal polyps (824). Release of preformed mediators from mast cells should foster tissue oedema while serine proteases will effect PAR receptors, degrade the extracellular matrix (ECM) and diminish barrier integrity. Interestingly, data are mixed as to whether mast cell numbers are increased in CRSwNP in comparison to either CRSsNP or even control tissues (542, 727, 874, 944-949). Nevertheless, functional studies suggest that mast cells in nasal polyps are much more active and may display a heightened sensitivity to external triggers in vivo (949). Overall however, the relative importance of mast cells in the pathogenesis of CRSwNP remains unclear. Targeted medications designed to inhibit upstream mast cell functions are an area of active research that may help elucidate their importance (950).
4.2.4.2.7. Cells, Plasma Cells and Immunoglobulins
Mucosal immunoglobulin secretion by cells of the B lymphocyte lineage is an important part of the adaptive immune response. In the nasal mucosa, B cells undergo proliferation, differentiation and immunoglobulin class switching to become mature plasma cells capable of substantial local antibody secretion. In overview, tonic secretion of sIgA works in concert with other innate protective factors and mucociliary flow to limit mucosal colonization without tissue-damaging inflammation (951). In general, this IgA is relatively low affinity, generated via a T-independent process, and secreted by extrafollicular B cells. In the case of an active breach of the respiratory mucosa, IgA secretion increases but it also receives help from IgG and a robust inflammatory response ensues. In general, this is high affinity IgA, T-dependent and generated by follicular B cells and plasma cells. IgM and IgD also play a role. IgD is the least understood imunoglobulin but interestingly, it is present in significant amounts in the respiratory mucosa (952). Although its precise role is still unclear, IgD exerts protective effects not only through antigen binding, but also its capacity to arm basophils with IgD highly reactive against respiratory bacteria (953). Basophils have recently been discovered to possess the capacity to function as antigen presenting cells by migrating back to lymphoid organs to initiate Th2 and B cell responses (954). Hence, IgD-activated basophils may initiate or enhance innate and adaptive responses both systemically and at the mucosa (952). IgE is mostly closely associated with the pathophysiology of allergic rhinitis but it plays several important physiologic roles as well including antigen presentation, increased mast cell survival, defense against viruses, bacteria, fungi and parasites and mucosal homeostasis (729, 940). In CRS, polyp homogenates demonstrate high levels of immunoglobulins, notably IgA, IgE and IgG, in comparison to CRSsNP and control tissues, apparently in response to bacterial and fungal antigens (542, 600, 786, 807, 921, 955-957). Levels in polyp homogenates do not correlate with levels in serum, suggesting that significant immunoglobulin synthesis occurs locally in the nasal mucosa (958-960). In parallel with these findings, high levels of B cells and plasma cells have been reported in nasal polyps in comparison to CRSsNP and control tissue (600, 874, 921). Evidence for a dysregulated adaptive B-cell immune response is further suggested by the presence of germinal center like follicles in nasal polyps (960) and the entire process is likely orchestrated by local proliferation and systemic recruitment of B cells (600, 602).
Elevations of tissue B cells, plasma cells and immunoglobulins are associated with CRSwNP.
In regard to elevated IgE in nasal polyps, levels have been shown to be independent of systemic atopy but they do correlate with the presence of IgE to staphylococcal superantigenic toxins (542). Approximately 50% of Caucasian CRSwNP and 20% of Chinese CRSwNP patients demonstrate local IgE to these toxins as well as a concomitant polyclonal IgE response to a diverse array of environmental antigens in polyp homogenates (542, 621). The presence of IgE to these toxins correlated with not only high levels of polyclonal IgE but also high tissue levels of ECP (eosinophil cationic protein) and co-morbid asthma (621). In regard the mechanism, studies of polyp explants exposed to staphylococcal superantigens revealed polyclonal T cell activation with a Th2 cytokine polarization (668, 670). In addition to pro-eosinophilic effects, this cytokine milieu should favour IgE production indirectly by triggering B cell class switching towards IgE production (596). Furthermore, staphylococcal protein A (SpA) has direct proliferative effects on B cells in vitro, possibly further driving the IgE process in nasal polyps (596). Very recent studies have demonstrated that the polyclonal IgE in nasal polyps is functional and can trigger mast cell de-granulation, suggesting a significant role for IgE in the pathophysiology of this subset of CRSwNP patients (961). The therapeutic potential of anti-IgE for nasal polyposis has been suggested (962) but trials have thus far been equivocal (963).
In regard to elevated IgA in nasal polyps, recent studies have implicated BAFF (also called BLyS or TNFSF13B), a cytokine of the TNF family favoring B cell proliferation and immunoglobulin class switching (600). High levels of BAFF are present in nasal polyp tissue in comparison of controls and CRSsNP tissue; moreover, the levels of BAFF correlate with the number of B cells in the nasal polyp (600). Transgenic BAFF mice develop autoimmune disorders (964); further studies in polyp homogenates demonstrated IgA and IgG anti-nuclear autoantibodies at locally elevated levels in nasal polyp tissue in the absence of systemic autoimmunity in some patients with CRSwNP (740). The presence of these autoantibodies was detected at higher frequency in the most recalcitrant patients who had undergone multiple revision surgical procedures, suggesting an autoimmune component in the most severe subset of CRSwNP.
The presence of both abundant class-switched immunoglobulins and available antigen is likely to play an important role in propagating the inflammatory response through antibody-mediated mechanisms (955). As noted in other sections of this review, CRSwNP is associated with increased infiltration of inflammatory effector cells including eosinophils, mast cells, macrophages and neutrophils, which de-granulate or phagocytose in response to immune complexes (874, 965). The potential impact of IgE and mast cell activation in CRSwNP was already noted. Similarly, IgA is an extremely potent trigger of eosinophilic degranulation and hence may be a key to local mediator release within polyp tissue as well (919). A potential role for IgD is CRS is thus far speculative, however the capacity to arm basophils is intriguing and this immunoglobulin may play a significant upstream role in fostering a Th2 cytokine milieu in nasal polyposis.
4.2.4.8. T Cells and cytokine patterns
Comparatively few studies have examined the topic of T cell activity in the nasal mucosa relative to the gut, skin and lower airways. In addition, many studies have been performed in vitro, and the in vivo factors mediating T cell responses, in particular Th polarization across mucosal barriers remains a subject of active research. In regard to CRS, the absence of a widely accepted animal model compounds the problem; hence, much of our understanding of T cell activity in nasal mucosa is based on extrapolation. In the immune response of the nose, dendritic cells (DCs) act as the initial antigen presenting cells (APCs) sampling and then presenting antigens to naïve T lymphocytes in draining lymph nodes or local lymph aggregates. Circulating basophils may also enter the tissue and serve along side or instead of resident DCs to function as APCs as well (966). Following antigen presentation, naïve CD4+ lymphocytes will differentiate into one of several T cell lineages, determining the nature of the adaptive immune response. The subsets include Th1 and Th2 as well as the more recently described Th17 and inducible T regulatory cells; each has distinct molecular, cellular and functional properties (967, 968). Other subsets have also been recently proposed, including Th9 and Th22, and more are likely to follow. In vitro studies indicate that for the Th1 subset, the key transcription factor is T-bet, the canonical cytokine is IFN-γ and the classical cellular infiltrate is macrophage-rich. Th1 responses are particularly effective against viruses and intracellular bacteria, including mycobacteria. For Th2, the transcription factor is GATA-3, the associated cytokines are IL-4, IL-5 and IL-13 and the cellular response eosinophilic. Th2 protective responses are geared against parasites, particularly those too large to undergo phagocytosis. For Th17, the transcription factor is RORc and the associated cytokine IL-17A and the cellular response classically neutrophilic. Extracellular bacteria, particularly Staphylococcus aureus (969), are prime targets. T regulatory cells are characterized by the transcription factor FOXP3 with the purpose of limiting excessive responses by the other lineage subsets. These differentiated effector T cells migrate into the mucosa where they re-encounter the same antigen, this time likely presented by both macrophages and DCs acting as APCs. The resultant binding of antigen to the T cell receptor (TCR) activates the cell, resulting in a cytokine release pattern characteristic for each Th subtype, mediating the appropriate effector response.
The in vivo factors that determine T cell differentiation are obviously critical, but currently somewhat speculative in the nasal mucosa. In general, the differentiation of naïve CD4+ cells into a particular lineage is the integration of multiple signals, including T-cell receptor strength, co-stimulatory and innate immune signals, and cytokine milieu (967, 968). This process is greatly influenced by crosstalk between epithelial cells (ECs) and the local DCs (601). ECs, as well as other resident innate cell types (mast cells, NK cells, macrophages, basophils, eosinophils), sense exogenous, primarily microbial agents via PAR, Toll receptor, NLR and other PRR leading to expression of various cytokines and chemokines as mentioned in the earlier sections. Cellular damage is also detected via DAMPs. Collectively, these resident cells are therefore able to sense both damage and danger and respond with the appropriate cytokine array, secondarily influencing the correct effector T cell response to address the particular challenge. In addition to these resident cells influencing DC polarization, it has recently been recognized that circulating innate lymphoid cells (ILCs) migrate to the site of stimulation and also play a role (970). They have been recognized separately in a number of tissues and thus have diverse names including NK cells, LTi cells, nuocytes, innate T cells, natural helper cells and CD34+ hemopoietic progenitor cells (835, 970-973). These ILCs are presumably responding to chemokine homing signals emanating from resident mucosal cells including ECs and are termed innate because they recognize foreign substances via PPRs rather than through TCRs or immunoglobulin. Capable of responding rapidly, ILCs function in a transitional effector cell role, bridging innate and adaptive immunity. Distinct subsets of ILCs have been proposed and the lineage relationship is not yet clear. Nevertheless upon stimulation, ILCs release cytokines that, among other functions, will influence DC polarization. While Th1 and Th17 ILCs have been described, in the case of CRSwNP, ILCs thus far identified are Th2 skewed, responding to epithelial cytokines such as IL-25, IL-33 and TSLP with the production of IL4, IL-5 and IL-13 (601, 974). Whether these cells play a role in CRSsNP is unclear, but earlier results suggest they may have a prominent role in CRSwNP since exceptionally high numbers of ILCs are found in nasal polyps (835, 973). No studies have been done on ILCs in Asian polyps or CF polyps, which might very well be distinct.
The collective cytokine response from resident cells and migrating ILCs is believed to be pivotal in shaping T cell differentiation. The typical in vivo T cell effector responses are mixed however, and the Th subtypes display some heterogeneity as well (975, 976). Nevertheless, the lineage subsets tend to be mutually inhibitory resulting in a degree of polarization to particular subsets at a site of action (968, 976). Under physiologic conditions, the typical adaptive response to harmless antigens is immunologic tolerance, with generation of Tregs and a baseline controlled Th2 response. Although the nasal mucosa has not been studied in vivo, this pattern presumably results from appropriate levels of TGF-β, IL-2, IL-4 and TSLP secretion influencing DC polarization (834, 968). TGF-β fosters Treg differentiation. IL-4 is required for Th2 differentiation in vitro but evidence suggests this restriction may be circumvented in vivo (977, 978). Alternatively, IL-4 may be secreted by resident mast cells or basophils. It is not known whether circulating innate immune cells play any significant role in baseline homeostasis. The net effect is a non-inflammatory response, primarily consisting of IgA secretion, which limits adherence of microbes to the epithelium (951).
Homeostasis across mucosal barriers is geared towards eliminating microbes and other antigens without tissuedamaging inflammation (951). When the mucosal barrier is breached, an appropriate protective immune response with some degree of inflammation must be generated, with ECs and other innate immune cells helping to guide the response. In the case of a protective Th1 response directed against intracellular microbes, ECs and other resident and infiltrating cells including NK cells, trigger IL-12, IL-18 and IFN-γ release, the essential cytokines fostering Th1 differentiation. When subsequently challenged by antigen, effector Th1 cells secrete large amounts of IFN-γ, TNF-α and TNF-β with several key protective effects: (25) macrophage activation with enhancement of phagocytic properties (14) B cell help and class switching to production of IgG subclasses with opsonizing and complement fixing capabilities (594) enhanced antigen presentation of macrophages and (625) local tissue inflammation and neutrophil activation (979).
Protective Th2 responses are directed against parasites, and cytokines such as TSLP, IL-33 and possibly IL-25 may play roles, with the net effect being a milieu favoring a much stronger skewing of Th2 T cell differentiation than seen under homeostatic conditions (834). Circulating ILCs likely contribute to the Th2 cytokine milieu as mentioned above (973). Basophils, mast cells and NKT cells (natural killer T cells) are possible sources of IL-4, which may be essential for the process as mentioned above (977). When subsequently challenged by antigen, Th2 effector cells secrete large amounts of Th2 cytokines IL-4, IL-5 and IL-13, which may drive more TSLP secretion by ECs, creating a positive feedback loop (977). The net protective effects of these Th2 cytokines includes (25) recruitment, activation and survival enhancement of eosinophils, in particular by IL-5 (14) immunoglobulin class switching to IgE and IgG4 via IL-4 and IL-13 (594) increased mucus production via IL-13 and (625) alternative macrophage activation by IL-4 and IL-13. IgE and IgA are capable of binding parasites, sterically inhibiting their ability to invade, but these immunoglobulins do not trigger phagocytosis or complement fixation. Mast cell binding to this surface IgE triggers de-granulation with release of inflammatory mediators and substances toxic to the parasites. Similarly, eosinophils may bind IgA with release of granules toxic to the parasites as well. Alternatively, high tissue IL-5 levels may also foster eosinophil degranulation in the absence of IgA. Mast cell and eosinophil degranulation trigger inflammation and some degree of tissue damage, which are both inevitable and necessary, but have long-term negative consequences. Lastly, alternative macrophage activation will trigger expression of macrophage mannose receptor (MMR) and secretion of cytokines that stimulate collagen synthesis and fibrosis. While these granuloma-forming activities may be protective in certain settings, they can have significantly negative effects on endorgan function.
In the case of a protective response against extracellular bacteria and fungi, Th17 responses are preferentially invoked via resident cell cytokine responses including IL-1β and IL-6 (968, 980). As mentioned above, TGF-β alone fosters Treg differentiation; however TGF-β together with IL-6 will foster Th17 differentiation and the presumed sources of IL-6 are macrophages, DCs and ECs (981). Th17 cells produce large amounts of IL-17A, IL-17F and IL-22 with several protective effects both directly and indirectly including (25) neutrophil recruitment (14) neutrophil activation (594) neutrophil proliferation and (625) innate antimicrobial production by airway epithelial cells (980).
In addition to the CD4+ helper T cell subsets discussed above, CD8+ cytotoxic T cells, natural killer (NK) cells, NKT and memory T cells also play significant roles in mucosal immunity. Naive CD8+ T cells differentiate and proliferate following exposure to antigen presented by DCs. CD4+T cells provide signals that amplify the process and may be absolutely essential in the case of some antigens. The net result is the generation of cytotoxic lymphocytes (CTLs) whose primary function is to eliminate intracellular microbes mainly by killing infected cells. Infected cells display microbial antigens on the surface together with class I MHC molecules, and this complex is recognized by the TCR. The infected cells undergo apoptosis from toxic granule exposure or via a ligand-receptor mediated process. CTLs are frequently localized to the epithelium; the TCRs of these lymphocytes often show limited diversity suggesting they have a restricted response repertoire and may be focused on commonly encountered luminal antigens. NK cells have a similar function to CTLs but their receptors are distinct from TCRs and they also do not need to undergo differentiation or maturation. They recognize stressed/infected cells via differential expression of a heterogeneous group of endogenous surface ligands rather than foreign antigen; the result is lysis of the stressed cell. They also secrete IFN-γ, which activates macrophages and fosters Th1 differentiation. NKT cells are a numerically small population of lymphocytes that have characteristics of both T cells and NK cells. They have TCRs but with limited variability, typically against lipid antigens, distinguishing them from typical T cells which only recognize protein antigens. They are also a source of IFN-γ. Memory lymphocytes are generated alongside the differentiation and maturation of the effector CTL and Th lineages and are actually the predominant T lymphocyte subset in nasal polyps (982)..These memory cells are present in the mucosa and respond to subsequent antigen challenge.
The role of T cells in chronic airway inflammation has been a subject of great interest since the discovery of Th1/Th2 paradigm 25 years ago; consequently most studies have focused on the CD4+ lineage subsets (983). Given the chronic inflammation that defines CRS, the presence of elevated levels of T cells in both CRSsNP and CRSwNP relative to control tissues is not surprising (620, 874, 984). It has been proposed however, that the various T cell effector lineages orchestrate distinct phenotypes of CRS (22, 620, 984). Establishing the predominant T effector pattern may therefore help determine pathophysiology, guide treatment, or even predict outcome. Early work in this area demonstrated elevations of both Th1 and Th2 cytokines in CRS, with higher levels of Th2 cytokines associated with atopy (524). Follow up studies failed to confirm this latter finding, indicating that Th2 cytokine levels were independent of atopy (900). Later studies began the actual process of separating disease phenotype and cytokine response. These results indicated that in Caucasians, CRSsNP is a skewed Th1 disorder, with relatively higher levels of IFN-γ while CRSwNP is a skewed Th2 disorder with relatively higher levels of IL-5 (620). In addition, CRSwNP had evidence for a relative lack of T regulatory function based on decreased FOXP3 expression (984, 985). Studies on Asian CRS tissues have yielded some differences and some similarities. Decreased Treg function with CRSwNP appears to be similar in both Asian and Caucasian polyps (22, 877). CRSsNP in Asians was shown to be relatively Th1 biased, similar to Caucasians as well (877). Asian CRSwNP patients demonstrated a Th1/Th17 cytokine bias, with less IL-5 than Caucasian polyps, consistent with the lower eosinophilic and higher neutrophilic tissue infiltration (22, 875, 877, 986, 987). Other investigators however, showed no differences between Asian CRSwNP and Caucasian CRSwNP with regard to IL-5 or eosinophilia but this has been interpreted to reflect wide variations in environmental and/or genetic factors across the continent (988, 989).
Asian and Western CRSwNP both exhibit low TGF-β and diminished Treg activity relative to CRSsNP
Recently, comparative expression analyses of the key canonical cytokines IFN- γ, IL-5 and IL-17 were performed in both Chinese and Belgian polyps. This is the most comprehensive study of its kind to date and it confirmed the Th2 bias in Western/Caucasian polyps and the Th1/Th17 bias in Chinese polyps (621). The study further revealed that a substantial proportion of Chinese polyps were negative for all 3 key cytokines, termed therefore KCN polyps (key cytokine negative). Most significantly, high IL-5, polyclonal IgE with IgE to staphylococcal exotoxins and comorbid asthma clustered in both groups (621). A later follow up study has associated the inflammatory cytokine pattern with bacterial colonization indicating that KCN polyps are associated with gram negative bacterial colonization while the smaller Th2 skewed subset of Chinese polyps is preferentially colonized by gram positive organisms (623). While the rate of Staphylococcus aureus colonization is much lower even in the IL-5 positive Chinese polyps, these results are in relative agreement with published findings in Caucasian CRSwNP patients further connecting this organism with Th2 cytokine expression (661). While these findings are interesting, it remains unclear whether the cytokine patterns can predict clinical phenotype or response to therapy. Despite differences in levels of inflammatory cytokines, low FOX3P expression appears to be characteristic of both Asian and Caucasian polyps patients indicating that diminished Treg activity may be a key factor in polyp formation (22, 984, 990).
NK, NKT and CD8+ T cells are relatively unstudied in CRS. NK cells are present and apparently elevated vs. control tissue in both CRSsNP and CRSwNP but any specific role in the disease process is unclear (731, 874, 982). Normal nasal mucosa demonstrates a ratio of CD4+ to CD8+ cells of approximately 2:1 (535, 991). In nasal polyps, relatively more CD8+ T-cells have been demonstrated but the implications for pathogenesis remain unclear (877, 982, 992). Studies on Asian CRSsNP patients also showed a higher proportion of CD8+ cells (877). Given the potential role of viruses and other intracellular pathogens in CRS in general and acute exacerbations in particular, further studies on NK, NKT and CD8+ cells may be quite important.
In summary, there is substantial evidence for (25) a down regulation of Treg activity in CRSwNP and (14) upregulation of Th 1, 2 and 17 in various forms of CRS. Current evidence indicates that CRSsNP tends to be a relatively Th1 biased disorder in both Caucasians and Asians. CRSwNP is Th2 biased in Caucasians while Th1/Th17 biased in Asians. CF nasal polyps are likely Th17 biased but this has not been directly assessed (981). While these studies represent data aggregates, individual patient outliers are present in each group and it remains to be demonstrated whether these outliers are distinct in terms of aetiology and clinical behavior.
Neutrophils are circulating immune effector cells with an established role in the early phagocytosis and killing of extracellular microbes. Recruitment to mucosal sites is typically driven by microbial stimulation of PRRs, with release of cytokines that trigger endothelial expression of selectins, integrin ligands and chemokines. The main chemokine fostering neutrophil recruitment in CRS appears to be IL-8, in part released by nasal epithelial cells in response to PAR2 stimulation (720). The role of the neutrophil in CRS remains unclear but the highest sinus tissue levels are seen in CF patients (862). For other forms of CRS, differences appear to depend on ethnicity as well as the presence or absence of nasal polyps. In Caucasians, neutrophilic infiltration can be demonstrated in CRS, with slightly lower levels observed in CRSsNP than in CRSwNP (620, 873, 874). In concert, studies have shown upregulation of IL-8 in both CRSwNP and CRSsNP (620, 933-935). Neutrophils did not appear to replace eosinophils in CRS mucosa, rather they were superimposed on the process; hence the term 'neutrophilic' rhinosinusitis was not considered completely appropriate for CRSsNP (874). Nevertheless, the degree of neutrophilic infiltrate was comparable between CRSsNP and CRSwNP as opposed to the eosinophilic infiltrate, which was significantly less in CRSsNP. As a corollary, it has been suggested that CRSsNP is more distinctly a neutrophilic process, while CRSwNP is more eosinophilic based on the relative degree of tissue infiltration (936). Furthermore, in the subpopulation of CRSwNP patients with relatively low eosinophilic infiltration, it has been suggested that neutrophils may be the major pathologic driver of disease, analogous to 'neutrophilic' asthma (19). In studies of polyps from Chinese patients, neutrophilic and eosinophilic infiltration appeared to be less than that seen in Caucasian polyps but the degree of eosinophilia was much more reduced, hence these polyps were relatively neutrophilic (22, 875). A later study on Chinese patients from a different region indicated that Asian CRSsNP patients were comparably much more neutrophilic than Asian CRSwNP patients (877). In the subset of Asian polyps that were non-eosinophilic however, significant neutrophilia was observed suggesting distinct underlying pathogenic processes within the CRSwNP group (877). Overall, it should be kept in mind that Asian polyps may be quite different in cellular and cytokine profile throughout the continent. Traditionally, neutrophils have been considered an acute response cell with a relatively short tissue half-life, therefore reasons for their accumulation in CRS are not completely clear. Recent studies have however, expanded the role of neutrophils beyond phagocytocis of extracellular organisms based in part on their diverse repertoire of effector molecules, which they express upon appropriate stimulation. In particular, neutrophils, may play a significant role in the resolution of inflammation as well as the pathology of the chronic inflammatory state (937). Chronic neutrophilic inflammation is observed in lung disorders such as COPD and CF, mediating extensive tissue injury and contributing to organ dysfunction. Neutrophil products include various proteolytic enzymes, which may alter the proteaseantiprotease balance triggering damage and remodeling. The excessive accumulation of neutrophils may be driven by the products derived from the breakdown of extracellular matrix, namely N-acetyl Pro-Gly-Pro (PGP) (938). PGP is normally metabolized but the process is impeded by cigarette smoke, with resulting inappropriate neutrophil accumulation in COPD (939). In CF lungs, low extracellular chloride levels, driven by the CFTR defect, has been proposed to diminish physiologic PGP breakdown (939). Whether these processes take place in CF polyps or neutrophilic CRS in general is unknown. Interestingly, this pathway links smoking with neutrophilic inflammation, a process suggested by a separate line of research in CRS (765). Nevertheless, they suggest a significant potential role for neutrophils in the pathophysiology of CRS and further suggest a molecular hypothesis for the negative effect of tobacco smoke on treatment outcomes.
4.2.4.6. Mast Cells
Mast cells are resident cells of the sinonasal mucosa with physiologic roles in innate immunity and wound healing (940). Activation of mast cells results in the release of pre-formed granules including histamine, serotonin, proteoglycans and serine proteases; in addition, de novo synthesis and secretion of various eicosanoids, chemokines and cytokines also takes place. Physiologic activation of mast cells in immune defense works in part through PPR stimulation (940). In nasal disease states, mast cell de-granulation has been most commonly implicated in allergic rhinitis via antigen-driven IgE cross-linking. In CRS, most interest has centered on a role for mast cells in nasal polyposis, in part due to the potential to induce, augment and maintain eosinophilic inflammation through IgE-dependent and IgE-independent processes (941, 942). In particular, polyp explant studies have demonstrated that mast cell de-granulation may be triggered directly by protein A (SpA), a staphylococcal surface protein (668). Mast cell prostaglandins have been implicated in Th2 lymphocyte recruitment and activation in nasal polyps (669). These results suggest that mast cells can activate Th2 lymphocytes independently of T-cell receptor activation, with attendant secretion of Th2 cytokines (943). Stem cell factor, secreted by epithelial cells, may be important in the recruitment of mast cells in nasal polyps (824). Release of preformed mediators from mast cells should foster tissue oedema while serine proteases will effect PAR receptors, degrade the extracellular matrix (ECM) and diminish barrier integrity. Interestingly, data are mixed as to whether mast cell numbers are increased in CRSwNP in comparison to either CRSsNP or even control tissues (542, 727, 874, 944-949). Nevertheless, functional studies suggest that mast cells in nasal polyps are much more active and may display a heightened sensitivity to external triggers in vivo (949). Overall however, the relative importance of mast cells in the pathogenesis of CRSwNP remains unclear. Targeted medications designed to inhibit upstream mast cell functions are an area of active research that may help elucidate their importance (950).
4.2.4.2.7. Cells, Plasma Cells and Immunoglobulins
Mucosal immunoglobulin secretion by cells of the B lymphocyte lineage is an important part of the adaptive immune response. In the nasal mucosa, B cells undergo proliferation, differentiation and immunoglobulin class switching to become mature plasma cells capable of substantial local antibody secretion. In overview, tonic secretion of sIgA works in concert with other innate protective factors and mucociliary flow to limit mucosal colonization without tissue-damaging inflammation (951). In general, this IgA is relatively low affinity, generated via a T-independent process, and secreted by extrafollicular B cells. In the case of an active breach of the respiratory mucosa, IgA secretion increases but it also receives help from IgG and a robust inflammatory response ensues. In general, this is high affinity IgA, T-dependent and generated by follicular B cells and plasma cells. IgM and IgD also play a role. IgD is the least understood imunoglobulin but interestingly, it is present in significant amounts in the respiratory mucosa (952). Although its precise role is still unclear, IgD exerts protective effects not only through antigen binding, but also its capacity to arm basophils with IgD highly reactive against respiratory bacteria (953). Basophils have recently been discovered to possess the capacity to function as antigen presenting cells by migrating back to lymphoid organs to initiate Th2 and B cell responses (954). Hence, IgD-activated basophils may initiate or enhance innate and adaptive responses both systemically and at the mucosa (952). IgE is mostly closely associated with the pathophysiology of allergic rhinitis but it plays several important physiologic roles as well including antigen presentation, increased mast cell survival, defense against viruses, bacteria, fungi and parasites and mucosal homeostasis (729, 940). In CRS, polyp homogenates demonstrate high levels of immunoglobulins, notably IgA, IgE and IgG, in comparison to CRSsNP and control tissues, apparently in response to bacterial and fungal antigens (542, 600, 786, 807, 921, 955-957). Levels in polyp homogenates do not correlate with levels in serum, suggesting that significant immunoglobulin synthesis occurs locally in the nasal mucosa (958-960). In parallel with these findings, high levels of B cells and plasma cells have been reported in nasal polyps in comparison to CRSsNP and control tissue (600, 874, 921). Evidence for a dysregulated adaptive B-cell immune response is further suggested by the presence of germinal center like follicles in nasal polyps (960) and the entire process is likely orchestrated by local proliferation and systemic recruitment of B cells (600, 602).
Elevations of tissue B cells, plasma cells and immunoglobulins are associated with CRSwNP.
In regard to elevated IgE in nasal polyps, levels have been shown to be independent of systemic atopy but they do correlate with the presence of IgE to staphylococcal superantigenic toxins (542). Approximately 50% of Caucasian CRSwNP and 20% of Chinese CRSwNP patients demonstrate local IgE to these toxins as well as a concomitant polyclonal IgE response to a diverse array of environmental antigens in polyp homogenates (542, 621). The presence of IgE to these toxins correlated with not only high levels of polyclonal IgE but also high tissue levels of ECP (eosinophil cationic protein) and co-morbid asthma (621). In regard the mechanism, studies of polyp explants exposed to staphylococcal superantigens revealed polyclonal T cell activation with a Th2 cytokine polarization (668, 670). In addition to pro-eosinophilic effects, this cytokine milieu should favour IgE production indirectly by triggering B cell class switching towards IgE production (596). Furthermore, staphylococcal protein A (SpA) has direct proliferative effects on B cells in vitro, possibly further driving the IgE process in nasal polyps (596). Very recent studies have demonstrated that the polyclonal IgE in nasal polyps is functional and can trigger mast cell de-granulation, suggesting a significant role for IgE in the pathophysiology of this subset of CRSwNP patients (961). The therapeutic potential of anti-IgE for nasal polyposis has been suggested (962) but trials have thus far been equivocal (963).
In regard to elevated IgA in nasal polyps, recent studies have implicated BAFF (also called BLyS or TNFSF13B), a cytokine of the TNF family favoring B cell proliferation and immunoglobulin class switching (600). High levels of BAFF are present in nasal polyp tissue in comparison of controls and CRSsNP tissue; moreover, the levels of BAFF correlate with the number of B cells in the nasal polyp (600). Transgenic BAFF mice develop autoimmune disorders (964); further studies in polyp homogenates demonstrated IgA and IgG anti-nuclear autoantibodies at locally elevated levels in nasal polyp tissue in the absence of systemic autoimmunity in some patients with CRSwNP (740). The presence of these autoantibodies was detected at higher frequency in the most recalcitrant patients who had undergone multiple revision surgical procedures, suggesting an autoimmune component in the most severe subset of CRSwNP.
The presence of both abundant class-switched immunoglobulins and available antigen is likely to play an important role in propagating the inflammatory response through antibody-mediated mechanisms (955). As noted in other sections of this review, CRSwNP is associated with increased infiltration of inflammatory effector cells including eosinophils, mast cells, macrophages and neutrophils, which de-granulate or phagocytose in response to immune complexes (874, 965). The potential impact of IgE and mast cell activation in CRSwNP was already noted. Similarly, IgA is an extremely potent trigger of eosinophilic degranulation and hence may be a key to local mediator release within polyp tissue as well (919). A potential role for IgD is CRS is thus far speculative, however the capacity to arm basophils is intriguing and this immunoglobulin may play a significant upstream role in fostering a Th2 cytokine milieu in nasal polyposis.
4.2.4.8. T Cells and cytokine patterns
Comparatively few studies have examined the topic of T cell activity in the nasal mucosa relative to the gut, skin and lower airways. In addition, many studies have been performed in vitro, and the in vivo factors mediating T cell responses, in particular Th polarization across mucosal barriers remains a subject of active research. In regard to CRS, the absence of a widely accepted animal model compounds the problem; hence, much of our understanding of T cell activity in nasal mucosa is based on extrapolation. In the immune response of the nose, dendritic cells (DCs) act as the initial antigen presenting cells (APCs) sampling and then presenting antigens to naïve T lymphocytes in draining lymph nodes or local lymph aggregates. Circulating basophils may also enter the tissue and serve along side or instead of resident DCs to function as APCs as well (966). Following antigen presentation, naïve CD4+ lymphocytes will differentiate into one of several T cell lineages, determining the nature of the adaptive immune response. The subsets include Th1 and Th2 as well as the more recently described Th17 and inducible T regulatory cells; each has distinct molecular, cellular and functional properties (967, 968). Other subsets have also been recently proposed, including Th9 and Th22, and more are likely to follow. In vitro studies indicate that for the Th1 subset, the key transcription factor is T-bet, the canonical cytokine is IFN-γ and the classical cellular infiltrate is macrophage-rich. Th1 responses are particularly effective against viruses and intracellular bacteria, including mycobacteria. For Th2, the transcription factor is GATA-3, the associated cytokines are IL-4, IL-5 and IL-13 and the cellular response eosinophilic. Th2 protective responses are geared against parasites, particularly those too large to undergo phagocytosis. For Th17, the transcription factor is RORc and the associated cytokine IL-17A and the cellular response classically neutrophilic. Extracellular bacteria, particularly Staphylococcus aureus (969), are prime targets. T regulatory cells are characterized by the transcription factor FOXP3 with the purpose of limiting excessive responses by the other lineage subsets. These differentiated effector T cells migrate into the mucosa where they re-encounter the same antigen, this time likely presented by both macrophages and DCs acting as APCs. The resultant binding of antigen to the T cell receptor (TCR) activates the cell, resulting in a cytokine release pattern characteristic for each Th subtype, mediating the appropriate effector response.
The in vivo factors that determine T cell differentiation are obviously critical, but currently somewhat speculative in the nasal mucosa. In general, the differentiation of naïve CD4+ cells into a particular lineage is the integration of multiple signals, including T-cell receptor strength, co-stimulatory and innate immune signals, and cytokine milieu (967, 968). This process is greatly influenced by crosstalk between epithelial cells (ECs) and the local DCs (601). ECs, as well as other resident innate cell types (mast cells, NK cells, macrophages, basophils, eosinophils), sense exogenous, primarily microbial agents via PAR, Toll receptor, NLR and other PRR leading to expression of various cytokines and chemokines as mentioned in the earlier sections. Cellular damage is also detected via DAMPs. Collectively, these resident cells are therefore able to sense both damage and danger and respond with the appropriate cytokine array, secondarily influencing the correct effector T cell response to address the particular challenge. In addition to these resident cells influencing DC polarization, it has recently been recognized that circulating innate lymphoid cells (ILCs) migrate to the site of stimulation and also play a role (970). They have been recognized separately in a number of tissues and thus have diverse names including NK cells, LTi cells, nuocytes, innate T cells, natural helper cells and CD34+ hemopoietic progenitor cells (835, 970-973). These ILCs are presumably responding to chemokine homing signals emanating from resident mucosal cells including ECs and are termed innate because they recognize foreign substances via PPRs rather than through TCRs or immunoglobulin. Capable of responding rapidly, ILCs function in a transitional effector cell role, bridging innate and adaptive immunity. Distinct subsets of ILCs have been proposed and the lineage relationship is not yet clear. Nevertheless upon stimulation, ILCs release cytokines that, among other functions, will influence DC polarization. While Th1 and Th17 ILCs have been described, in the case of CRSwNP, ILCs thus far identified are Th2 skewed, responding to epithelial cytokines such as IL-25, IL-33 and TSLP with the production of IL4, IL-5 and IL-13 (601, 974). Whether these cells play a role in CRSsNP is unclear, but earlier results suggest they may have a prominent role in CRSwNP since exceptionally high numbers of ILCs are found in nasal polyps (835, 973). No studies have been done on ILCs in Asian polyps or CF polyps, which might very well be distinct.
The collective cytokine response from resident cells and migrating ILCs is believed to be pivotal in shaping T cell differentiation. The typical in vivo T cell effector responses are mixed however, and the Th subtypes display some heterogeneity as well (975, 976). Nevertheless, the lineage subsets tend to be mutually inhibitory resulting in a degree of polarization to particular subsets at a site of action (968, 976). Under physiologic conditions, the typical adaptive response to harmless antigens is immunologic tolerance, with generation of Tregs and a baseline controlled Th2 response. Although the nasal mucosa has not been studied in vivo, this pattern presumably results from appropriate levels of TGF-β, IL-2, IL-4 and TSLP secretion influencing DC polarization (834, 968). TGF-β fosters Treg differentiation. IL-4 is required for Th2 differentiation in vitro but evidence suggests this restriction may be circumvented in vivo (977, 978). Alternatively, IL-4 may be secreted by resident mast cells or basophils. It is not known whether circulating innate immune cells play any significant role in baseline homeostasis. The net effect is a non-inflammatory response, primarily consisting of IgA secretion, which limits adherence of microbes to the epithelium (951).
Homeostasis across mucosal barriers is geared towards eliminating microbes and other antigens without tissuedamaging inflammation (951). When the mucosal barrier is breached, an appropriate protective immune response with some degree of inflammation must be generated, with ECs and other innate immune cells helping to guide the response. In the case of a protective Th1 response directed against intracellular microbes, ECs and other resident and infiltrating cells including NK cells, trigger IL-12, IL-18 and IFN-γ release, the essential cytokines fostering Th1 differentiation. When subsequently challenged by antigen, effector Th1 cells secrete large amounts of IFN-γ, TNF-α and TNF-β with several key protective effects: (25) macrophage activation with enhancement of phagocytic properties (14) B cell help and class switching to production of IgG subclasses with opsonizing and complement fixing capabilities (594) enhanced antigen presentation of macrophages and (625) local tissue inflammation and neutrophil activation (979).
Protective Th2 responses are directed against parasites, and cytokines such as TSLP, IL-33 and possibly IL-25 may play roles, with the net effect being a milieu favoring a much stronger skewing of Th2 T cell differentiation than seen under homeostatic conditions (834). Circulating ILCs likely contribute to the Th2 cytokine milieu as mentioned above (973). Basophils, mast cells and NKT cells (natural killer T cells) are possible sources of IL-4, which may be essential for the process as mentioned above (977). When subsequently challenged by antigen, Th2 effector cells secrete large amounts of Th2 cytokines IL-4, IL-5 and IL-13, which may drive more TSLP secretion by ECs, creating a positive feedback loop (977). The net protective effects of these Th2 cytokines includes (25) recruitment, activation and survival enhancement of eosinophils, in particular by IL-5 (14) immunoglobulin class switching to IgE and IgG4 via IL-4 and IL-13 (594) increased mucus production via IL-13 and (625) alternative macrophage activation by IL-4 and IL-13. IgE and IgA are capable of binding parasites, sterically inhibiting their ability to invade, but these immunoglobulins do not trigger phagocytosis or complement fixation. Mast cell binding to this surface IgE triggers de-granulation with release of inflammatory mediators and substances toxic to the parasites. Similarly, eosinophils may bind IgA with release of granules toxic to the parasites as well. Alternatively, high tissue IL-5 levels may also foster eosinophil degranulation in the absence of IgA. Mast cell and eosinophil degranulation trigger inflammation and some degree of tissue damage, which are both inevitable and necessary, but have long-term negative consequences. Lastly, alternative macrophage activation will trigger expression of macrophage mannose receptor (MMR) and secretion of cytokines that stimulate collagen synthesis and fibrosis. While these granuloma-forming activities may be protective in certain settings, they can have significantly negative effects on endorgan function.
In the case of a protective response against extracellular bacteria and fungi, Th17 responses are preferentially invoked via resident cell cytokine responses including IL-1β and IL-6 (968, 980). As mentioned above, TGF-β alone fosters Treg differentiation; however TGF-β together with IL-6 will foster Th17 differentiation and the presumed sources of IL-6 are macrophages, DCs and ECs (981). Th17 cells produce large amounts of IL-17A, IL-17F and IL-22 with several protective effects both directly and indirectly including (25) neutrophil recruitment (14) neutrophil activation (594) neutrophil proliferation and (625) innate antimicrobial production by airway epithelial cells (980).
In addition to the CD4+ helper T cell subsets discussed above, CD8+ cytotoxic T cells, natural killer (NK) cells, NKT and memory T cells also play significant roles in mucosal immunity. Naive CD8+ T cells differentiate and proliferate following exposure to antigen presented by DCs. CD4+T cells provide signals that amplify the process and may be absolutely essential in the case of some antigens. The net result is the generation of cytotoxic lymphocytes (CTLs) whose primary function is to eliminate intracellular microbes mainly by killing infected cells. Infected cells display microbial antigens on the surface together with class I MHC molecules, and this complex is recognized by the TCR. The infected cells undergo apoptosis from toxic granule exposure or via a ligand-receptor mediated process. CTLs are frequently localized to the epithelium; the TCRs of these lymphocytes often show limited diversity suggesting they have a restricted response repertoire and may be focused on commonly encountered luminal antigens. NK cells have a similar function to CTLs but their receptors are distinct from TCRs and they also do not need to undergo differentiation or maturation. They recognize stressed/infected cells via differential expression of a heterogeneous group of endogenous surface ligands rather than foreign antigen; the result is lysis of the stressed cell. They also secrete IFN-γ, which activates macrophages and fosters Th1 differentiation. NKT cells are a numerically small population of lymphocytes that have characteristics of both T cells and NK cells. They have TCRs but with limited variability, typically against lipid antigens, distinguishing them from typical T cells which only recognize protein antigens. They are also a source of IFN-γ. Memory lymphocytes are generated alongside the differentiation and maturation of the effector CTL and Th lineages and are actually the predominant T lymphocyte subset in nasal polyps (982)..These memory cells are present in the mucosa and respond to subsequent antigen challenge.
The role of T cells in chronic airway inflammation has been a subject of great interest since the discovery of Th1/Th2 paradigm 25 years ago; consequently most studies have focused on the CD4+ lineage subsets (983). Given the chronic inflammation that defines CRS, the presence of elevated levels of T cells in both CRSsNP and CRSwNP relative to control tissues is not surprising (620, 874, 984). It has been proposed however, that the various T cell effector lineages orchestrate distinct phenotypes of CRS (22, 620, 984). Establishing the predominant T effector pattern may therefore help determine pathophysiology, guide treatment, or even predict outcome. Early work in this area demonstrated elevations of both Th1 and Th2 cytokines in CRS, with higher levels of Th2 cytokines associated with atopy (524). Follow up studies failed to confirm this latter finding, indicating that Th2 cytokine levels were independent of atopy (900). Later studies began the actual process of separating disease phenotype and cytokine response. These results indicated that in Caucasians, CRSsNP is a skewed Th1 disorder, with relatively higher levels of IFN-γ while CRSwNP is a skewed Th2 disorder with relatively higher levels of IL-5 (620). In addition, CRSwNP had evidence for a relative lack of T regulatory function based on decreased FOXP3 expression (984, 985). Studies on Asian CRS tissues have yielded some differences and some similarities. Decreased Treg function with CRSwNP appears to be similar in both Asian and Caucasian polyps (22, 877). CRSsNP in Asians was shown to be relatively Th1 biased, similar to Caucasians as well (877). Asian CRSwNP patients demonstrated a Th1/Th17 cytokine bias, with less IL-5 than Caucasian polyps, consistent with the lower eosinophilic and higher neutrophilic tissue infiltration (22, 875, 877, 986, 987). Other investigators however, showed no differences between Asian CRSwNP and Caucasian CRSwNP with regard to IL-5 or eosinophilia but this has been interpreted to reflect wide variations in environmental and/or genetic factors across the continent (988, 989).
Asian and Western CRSwNP both exhibit low TGF-β and diminished Treg activity relative to CRSsNP
Recently, comparative expression analyses of the key canonical cytokines IFN- γ, IL-5 and IL-17 were performed in both Chinese and Belgian polyps. This is the most comprehensive study of its kind to date and it confirmed the Th2 bias in Western/Caucasian polyps and the Th1/Th17 bias in Chinese polyps (621). The study further revealed that a substantial proportion of Chinese polyps were negative for all 3 key cytokines, termed therefore KCN polyps (key cytokine negative). Most significantly, high IL-5, polyclonal IgE with IgE to staphylococcal exotoxins and comorbid asthma clustered in both groups (621). A later follow up study has associated the inflammatory cytokine pattern with bacterial colonization indicating that KCN polyps are associated with gram negative bacterial colonization while the smaller Th2 skewed subset of Chinese polyps is preferentially colonized by gram positive organisms (623). While the rate of Staphylococcus aureus colonization is much lower even in the IL-5 positive Chinese polyps, these results are in relative agreement with published findings in Caucasian CRSwNP patients further connecting this organism with Th2 cytokine expression (661). While these findings are interesting, it remains unclear whether the cytokine patterns can predict clinical phenotype or response to therapy. Despite differences in levels of inflammatory cytokines, low FOX3P expression appears to be characteristic of both Asian and Caucasian polyps patients indicating that diminished Treg activity may be a key factor in polyp formation (22, 984, 990).
NK, NKT and CD8+ T cells are relatively unstudied in CRS. NK cells are present and apparently elevated vs. control tissue in both CRSsNP and CRSwNP but any specific role in the disease process is unclear (731, 874, 982). Normal nasal mucosa demonstrates a ratio of CD4+ to CD8+ cells of approximately 2:1 (535, 991). In nasal polyps, relatively more CD8+ T-cells have been demonstrated but the implications for pathogenesis remain unclear (877, 982, 992). Studies on Asian CRSsNP patients also showed a higher proportion of CD8+ cells (877). Given the potential role of viruses and other intracellular pathogens in CRS in general and acute exacerbations in particular, further studies on NK, NKT and CD8+ cells may be quite important.
In summary, there is substantial evidence for (25) a down regulation of Treg activity in CRSwNP and (14) upregulation of Th 1, 2 and 17 in various forms of CRS. Current evidence indicates that CRSsNP tends to be a relatively Th1 biased disorder in both Caucasians and Asians. CRSwNP is Th2 biased in Caucasians while Th1/Th17 biased in Asians. CF nasal polyps are likely Th17 biased but this has not been directly assessed (981). While these studies represent data aggregates, individual patient outliers are present in each group and it remains to be demonstrated whether these outliers are distinct in terms of aetiology and clinical behavior.
4.2.4.9. Remodeling
Tissue remodeling refers to modifications of the normal composition and structural organization of tissues, typically in response to stress such as chronic inflammation. Characteristic patterns of airway remodeling have been associated with several chronic inflammatory lower airway disorders including cystic fibrosis, pulmonary fibrosis, COPD and asthma (993). Remodeling also takes place in the upper airway when subjected to chronic inflammation such as seen in allergic rhinitis and CRS with changes that include fibrosis, epithelial alterations, basement membrane thickening, goblet cell hyperplasia, sub-epithelial oedema and inflammatory cell infiltrates (985, 994). In general, the histopathologic changes have been likened most closely to those observed in asthma (701, 994). Recent reports indicate that the lower airway epithelium and underlying cells function as a unit, termed the epithelialmesenchymal unit (EMTU); structural and functional defects in the airway epithelium in asthma are proposed to trigger persistent epithelial activation with secondary, and ultimately irreversible changes in the underlying tissues (768, 995). Studies in the upper airway have begun to suggest that similar pathways may be operative. Areas of hyperplasia and sloughing are apparent in CRSwNP epithelium (988, 996). Other studies suggest that diminished epithelial healing, weaker mechanical barrier and diminished innate antimicrobial secretion may be characteristic of CRSwNP (25, 782). Increased ion transport and higher rates of ion permeability have been observed in nasal polyp epithelia supporting this concept (784-786)..Taken together, these studies suggest the hypothesis that a permissive, relatively vulnerable epithelial barrier in CRS results in secondary changes in the underlying mucosal tissues.
Remodeling of the extracellular matrix (ECM) of the lamina propria in CRS patients has been extensively studied and somewhat distinct remodeling patterns have been associated with subsets of disease. The ECM is a network of collagenous and non-collagenous structures that surround cells in the airway and affect many aspects of cellular behavior including migration, differentiation, survival and proliferation (993). PDGF is one factor that has been implicated in lower airway ECM remodeling and it may play a role in the upper airway of CRS patients with asthma as well (997). In CRS however, the ECM is grossly characterized by areas of oedema and fibrosis, with the latter dominating in CRSsNP and the former dominating in CRSwNP (998). The precise molecular factors mediating this differential remodeling pattern are not completely clear, but current evidence suggests a key role for the pleotropic cytokine TGF-β. Although not all studies agree (859), low levels of TGF-β have been demonstrated in CRSwNP and high levels in CRSsNP (984). TGF-β modulates ECM deposition in the airway (999) and it has been suggested that low levels in CRSwNP contribute to decreased tissue repair and collagen formation with secondary albumin deposition and tissue oedema, while high levels in CRSsNP mediate basement membrane thickening, excessive collagen deposition and fibrosis (990, 1000). TGF-β also has an established role in Treg differentiation as mentioned earlier. It should be noted that low Treg activity and low TGF-β are two factors that appear consistent in both Asian and Caucasian polyps despite clear differences in inflammation, suggesting a key, possibly integrated role in polyp formation (594). In regard CRSsNP, a very recent study focused on early stage disease, suggested that increased TGF-β is present prior to the onset of a significant inflammatory response (1001). Overall, these findings give credence to the hypothesis that CRS is primarily a remodeling disease, rather than an inflammatory disorder, best characterized and possibly best treated based on remodeling patterns (594).
The ECM is dynamic, reflecting the net balance of synthesis and degradation that is regulated, in part, by the actions of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) (1002). It has been proposed that an imbalance between these factors, mediated by TGF-β, triggers the oedema seen in CRSwNP (594). This hypothesis is supported by data suggesting differential expression levels of MMPs and TIMPs in CRSwNP when compared to CRSsNP and control tissue (622, 775, 990, 1003-1005). In addition, extracellular matrix metalloproteinase inducer (EMMPRIN) is also elevated in CRSwNP as opposed to controls, suggesting high levels of ECM degradation in polyps (811). Lastly, a recent ex vivo study suggested that S. aureus may promote polyposis by altering the MMP/TIMP milieu (672). While these studies suggest a role for MMPs and TIMPS in CRS remodeling in general and polyp formation in particular, further studies are necessary to elucidate a clear molecular pathway of disease pathogenesis. Asian and Western CRSwNP exhibit similar remodeling patterns of oedema and decreased tissue collagen deposition.
Angiogenic factors have also been associated with upper airway remodeling of the lamina propria in CRS, in particular CRSwNP, suggesting that angiogenesis may be a driving force in polyp formation. Angiogenin, a factor that induces blood vessel formation, has been associated with CRSwNP (1006). Vascular endothelial growth factor (VEGF), a key protein that modulates both angiogenesis and vascular permeability, is much more highly expressed in nasal polyp tissue than in CRSsNP or control tissues (1007-1009).Expression is seen primarily in the epithelium where it is believed to trigger epithelial hyperplasia (1009). Endothelial expression of VEGF has been hypothesized to mediate the profound oedema seen in CRSwNP tissues (1010). The pathophysiological trigger for these angiogenic factors is unclear, but relative hypoxia has been demonstrated in the maxillary sinuses of CRS patients (1011). Hypoxia is a potent inducer of VEGF from nasal fibroblasts in vitro (1012, 1013), likely acting through hypoxia-inducible factor (HIF-1α) (1014). This suggested the hypothesis that hypoxia, in part through ostiomeatal complex (OMC) occlusion, drives HIF-α expression secondarily triggering VEGF, TGF-β, nitric oxide synthetase, MMPs and IL-8 (626, 627, 1015, 1016) support of this hypothesis, microarray analysis demonstrated substantial up-regulation of HIF-α in non-eosinophilic polyps in comparison to control tissue (1017). It should be kept in mind however, that VEGF appears to upregulated in both eosinophilic and non-eosinophilic polyps but not CRSsNP. The latter is a disease more closely associated with OMC obstruction and presumably, hypoxia (625). The high blood flow to the nose and paranasal sinuses would seem to limit actual tissue hypoxia in CRS. Moreover, one would anticipate an extremely low polyp recurrence rate following aggressive surgery, if hypoxia were the primary driver of angiogenesis and subsequent polyp formation. Lastly, and perhaps most importantly, carefully performed histologic studies have failed to demonstrate that angiogenesis, regardless of the inciting agent, is a significant driving force in polyp growth (1018). This report suggests that the rate of angiogenesis required to meet the needs of the polyp are relatively low and can be driven by metabolic or mechanical factors, rather than being an integral part of the pathology as it is in neoplastic disease (1019). Components of the coagulation cascade have been implicated in CRS pathogenesis, primarily in regard to effects on tissue remodeling. Airway inflammation is associated with increased vascular permeability and leakage of plasma proteins into the extravascular space. Thrombin levels are significantly increased in the nasal secretions of patients with CRSwNP and asthma and it was proposed that this results in increased VEGF secretion from epithelial cells via a PAR-1 receptor pathway (1020). In addition, fibrinolytic components have been associated with CRS. Plasminogen activators such as uPA are elevated in CRSwNP tissues compared to controls and CRSsNP (1021). Levels of the uPA inhibitor plasminogen activator inhibitor-1 (PAI-1) were elevated in CRSsNP and this correlated closely with TGF-β levels suggesting a mechanistic link (1021). Further studies will be necessary to establish the clinical relevance to ECM changes seen in CRS.
Remodeling of the underlying bone has also been observed in CRS (1022) and the presence of remodeled osteitic bone has been proposed as an explanation for persistent disease (1023, 1024). The mechanism for this process remains unclear and no study has yet recovered microorganisms from the bone of CRS patients. Nevertheless, non-infectious inflammatory cytokines may drive bone and tissue remodeling in a wide array of disorders. In particular, the cytokines osteopontin (OPN) and periostin (POSTN) are members of a family of recently described tissue remodeling proteins (1025) that may be relevant to CRS. OPN has been implicated in both bone remodeling (1026) and Th2 airway inflammation (1027) in humans and studies have demonstrated particularly high levels in CRSwNP (826). A study has also suggested that OPN may modulate ECM deposition in CRSwNP, perhaps in relationship to TGF-β (1028). POSTN, also called osteoblastic-specific factor 2, has an established role in bone formation and is also up-regulated in CRSwNP (1028, 1029). In summary, while these cytokines are possible candidates mediating the bone remodeling observed in CRS, it remains unclear whether this process plays a clinically significant role in CRS pathogenesis (1030).
Mucus secretion with goblet cell and glandular hyperplasia are other features of upper airway remodeling in CRS, with changes in both the quantity and viscosity of the mucus (1031, 1032). These changes are likely mediated by cytokines including TNF-α, IL-8 and IL-13 (1033). Glandular hyperplasia and hypertrophy have been primarily associated with CRSsNP (998, 1034). MUC5AC and MUC5B are the main secreted mucins in the human airway, with MUC5A being produced primarily by goblet cells (1035). Differential expression of mucin genes is observed in CF, CRSsNP, CRSwNP and antrochonal polyps (1032, 1035, 1036). These mucins ultimately affect viscosity presumably accounting for the thin, watery mucus typical of CRSwNP and thick mucus observed in CF (1032). It has been suggested that the positive effects of longterm macrolides for CRS seen in some studies (16) may in part, reflect reversal of pathologic increases in mucus viscosity (1037). Practically speaking however, there are over 20 mucin genes and a wide range of factors likely influences production in the individual patient (1038-1040).
Tissue remodeling refers to modifications of the normal composition and structural organization of tissues, typically in response to stress such as chronic inflammation. Characteristic patterns of airway remodeling have been associated with several chronic inflammatory lower airway disorders including cystic fibrosis, pulmonary fibrosis, COPD and asthma (993). Remodeling also takes place in the upper airway when subjected to chronic inflammation such as seen in allergic rhinitis and CRS with changes that include fibrosis, epithelial alterations, basement membrane thickening, goblet cell hyperplasia, sub-epithelial oedema and inflammatory cell infiltrates (985, 994). In general, the histopathologic changes have been likened most closely to those observed in asthma (701, 994). Recent reports indicate that the lower airway epithelium and underlying cells function as a unit, termed the epithelialmesenchymal unit (EMTU); structural and functional defects in the airway epithelium in asthma are proposed to trigger persistent epithelial activation with secondary, and ultimately irreversible changes in the underlying tissues (768, 995). Studies in the upper airway have begun to suggest that similar pathways may be operative. Areas of hyperplasia and sloughing are apparent in CRSwNP epithelium (988, 996). Other studies suggest that diminished epithelial healing, weaker mechanical barrier and diminished innate antimicrobial secretion may be characteristic of CRSwNP (25, 782). Increased ion transport and higher rates of ion permeability have been observed in nasal polyp epithelia supporting this concept (784-786)..Taken together, these studies suggest the hypothesis that a permissive, relatively vulnerable epithelial barrier in CRS results in secondary changes in the underlying mucosal tissues.
Remodeling of the extracellular matrix (ECM) of the lamina propria in CRS patients has been extensively studied and somewhat distinct remodeling patterns have been associated with subsets of disease. The ECM is a network of collagenous and non-collagenous structures that surround cells in the airway and affect many aspects of cellular behavior including migration, differentiation, survival and proliferation (993). PDGF is one factor that has been implicated in lower airway ECM remodeling and it may play a role in the upper airway of CRS patients with asthma as well (997). In CRS however, the ECM is grossly characterized by areas of oedema and fibrosis, with the latter dominating in CRSsNP and the former dominating in CRSwNP (998). The precise molecular factors mediating this differential remodeling pattern are not completely clear, but current evidence suggests a key role for the pleotropic cytokine TGF-β. Although not all studies agree (859), low levels of TGF-β have been demonstrated in CRSwNP and high levels in CRSsNP (984). TGF-β modulates ECM deposition in the airway (999) and it has been suggested that low levels in CRSwNP contribute to decreased tissue repair and collagen formation with secondary albumin deposition and tissue oedema, while high levels in CRSsNP mediate basement membrane thickening, excessive collagen deposition and fibrosis (990, 1000). TGF-β also has an established role in Treg differentiation as mentioned earlier. It should be noted that low Treg activity and low TGF-β are two factors that appear consistent in both Asian and Caucasian polyps despite clear differences in inflammation, suggesting a key, possibly integrated role in polyp formation (594). In regard CRSsNP, a very recent study focused on early stage disease, suggested that increased TGF-β is present prior to the onset of a significant inflammatory response (1001). Overall, these findings give credence to the hypothesis that CRS is primarily a remodeling disease, rather than an inflammatory disorder, best characterized and possibly best treated based on remodeling patterns (594).
The ECM is dynamic, reflecting the net balance of synthesis and degradation that is regulated, in part, by the actions of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) (1002). It has been proposed that an imbalance between these factors, mediated by TGF-β, triggers the oedema seen in CRSwNP (594). This hypothesis is supported by data suggesting differential expression levels of MMPs and TIMPs in CRSwNP when compared to CRSsNP and control tissue (622, 775, 990, 1003-1005). In addition, extracellular matrix metalloproteinase inducer (EMMPRIN) is also elevated in CRSwNP as opposed to controls, suggesting high levels of ECM degradation in polyps (811). Lastly, a recent ex vivo study suggested that S. aureus may promote polyposis by altering the MMP/TIMP milieu (672). While these studies suggest a role for MMPs and TIMPS in CRS remodeling in general and polyp formation in particular, further studies are necessary to elucidate a clear molecular pathway of disease pathogenesis. Asian and Western CRSwNP exhibit similar remodeling patterns of oedema and decreased tissue collagen deposition.
Angiogenic factors have also been associated with upper airway remodeling of the lamina propria in CRS, in particular CRSwNP, suggesting that angiogenesis may be a driving force in polyp formation. Angiogenin, a factor that induces blood vessel formation, has been associated with CRSwNP (1006). Vascular endothelial growth factor (VEGF), a key protein that modulates both angiogenesis and vascular permeability, is much more highly expressed in nasal polyp tissue than in CRSsNP or control tissues (1007-1009).Expression is seen primarily in the epithelium where it is believed to trigger epithelial hyperplasia (1009). Endothelial expression of VEGF has been hypothesized to mediate the profound oedema seen in CRSwNP tissues (1010). The pathophysiological trigger for these angiogenic factors is unclear, but relative hypoxia has been demonstrated in the maxillary sinuses of CRS patients (1011). Hypoxia is a potent inducer of VEGF from nasal fibroblasts in vitro (1012, 1013), likely acting through hypoxia-inducible factor (HIF-1α) (1014). This suggested the hypothesis that hypoxia, in part through ostiomeatal complex (OMC) occlusion, drives HIF-α expression secondarily triggering VEGF, TGF-β, nitric oxide synthetase, MMPs and IL-8 (626, 627, 1015, 1016) support of this hypothesis, microarray analysis demonstrated substantial up-regulation of HIF-α in non-eosinophilic polyps in comparison to control tissue (1017). It should be kept in mind however, that VEGF appears to upregulated in both eosinophilic and non-eosinophilic polyps but not CRSsNP. The latter is a disease more closely associated with OMC obstruction and presumably, hypoxia (625). The high blood flow to the nose and paranasal sinuses would seem to limit actual tissue hypoxia in CRS. Moreover, one would anticipate an extremely low polyp recurrence rate following aggressive surgery, if hypoxia were the primary driver of angiogenesis and subsequent polyp formation. Lastly, and perhaps most importantly, carefully performed histologic studies have failed to demonstrate that angiogenesis, regardless of the inciting agent, is a significant driving force in polyp growth (1018). This report suggests that the rate of angiogenesis required to meet the needs of the polyp are relatively low and can be driven by metabolic or mechanical factors, rather than being an integral part of the pathology as it is in neoplastic disease (1019). Components of the coagulation cascade have been implicated in CRS pathogenesis, primarily in regard to effects on tissue remodeling. Airway inflammation is associated with increased vascular permeability and leakage of plasma proteins into the extravascular space. Thrombin levels are significantly increased in the nasal secretions of patients with CRSwNP and asthma and it was proposed that this results in increased VEGF secretion from epithelial cells via a PAR-1 receptor pathway (1020). In addition, fibrinolytic components have been associated with CRS. Plasminogen activators such as uPA are elevated in CRSwNP tissues compared to controls and CRSsNP (1021). Levels of the uPA inhibitor plasminogen activator inhibitor-1 (PAI-1) were elevated in CRSsNP and this correlated closely with TGF-β levels suggesting a mechanistic link (1021). Further studies will be necessary to establish the clinical relevance to ECM changes seen in CRS.
Remodeling of the underlying bone has also been observed in CRS (1022) and the presence of remodeled osteitic bone has been proposed as an explanation for persistent disease (1023, 1024). The mechanism for this process remains unclear and no study has yet recovered microorganisms from the bone of CRS patients. Nevertheless, non-infectious inflammatory cytokines may drive bone and tissue remodeling in a wide array of disorders. In particular, the cytokines osteopontin (OPN) and periostin (POSTN) are members of a family of recently described tissue remodeling proteins (1025) that may be relevant to CRS. OPN has been implicated in both bone remodeling (1026) and Th2 airway inflammation (1027) in humans and studies have demonstrated particularly high levels in CRSwNP (826). A study has also suggested that OPN may modulate ECM deposition in CRSwNP, perhaps in relationship to TGF-β (1028). POSTN, also called osteoblastic-specific factor 2, has an established role in bone formation and is also up-regulated in CRSwNP (1028, 1029). In summary, while these cytokines are possible candidates mediating the bone remodeling observed in CRS, it remains unclear whether this process plays a clinically significant role in CRS pathogenesis (1030).
Mucus secretion with goblet cell and glandular hyperplasia are other features of upper airway remodeling in CRS, with changes in both the quantity and viscosity of the mucus (1031, 1032). These changes are likely mediated by cytokines including TNF-α, IL-8 and IL-13 (1033). Glandular hyperplasia and hypertrophy have been primarily associated with CRSsNP (998, 1034). MUC5AC and MUC5B are the main secreted mucins in the human airway, with MUC5A being produced primarily by goblet cells (1035). Differential expression of mucin genes is observed in CF, CRSsNP, CRSwNP and antrochonal polyps (1032, 1035, 1036). These mucins ultimately affect viscosity presumably accounting for the thin, watery mucus typical of CRSwNP and thick mucus observed in CF (1032). It has been suggested that the positive effects of longterm macrolides for CRS seen in some studies (16) may in part, reflect reversal of pathologic increases in mucus viscosity (1037). Practically speaking however, there are over 20 mucin genes and a wide range of factors likely influences production in the individual patient (1038-1040).
4.2.4.10. Eicosanoids and the Arachidonic acid
pathway
Eicosanoids are signaling molecules with immunologic and inflammatory properties generated by oxidative metabolism of arachidonic acid (AA) (1041, 1042). Disturbances in this pathway have been most closely associated with aspirin-sensitive nasal polyposis, but abnormalities have also been suggested to potentially underlie aspirin-tolerant CRSwNP as well. There are several families of classical eicosanoids with distinct properties: (25) leukotrienes (14), prostaglandins (PGD2, PGE2 and PGF2) (594); prostacyclin (PGI2); and thromboxane (TXA2) (625). Leukotrienes are generated by lipoxygenase (5-LO) activity, while the other 3 are generated by cyclooxygenase enzyme (Cox-1 and Cox-2) activity. Also relevant are the lipoxans, technically termed non-classical eicosanoids, which are generated by 12/15 lipoxygenase (12/15-LO) activity. In general, lipoxans and PGE2 have anti-inflammatory effects while the rest are all proinflammatory.
Leukotriene formation requires 5-LO activity that gives rise to the LTA4 precursor; subsequent enzyme activity results in the production of LTB4, LTC4, LTD4 and LTE4. The latter three are known as the cysteinyl leukotrienes, formerly termed slow reacting substance of anaphylaxis (SRSA), and require leukotriene C4 synthase activity (LTC4 synthase). Genetic polymorphisms in LTC4 synthase have been associated with CRSwNP in some studies (1043) and it has been suggested that this enzyme may be the engine of aspirin intolerance (1044, 1045). The primary sources of leukotrienes in the airway mucosa are mast cells and eosinophils, with effects including increased vascular permeability, vasodilation, leukocyte chemotaxis, broncho-constriction and mucus secretion. Leukotrienes have a short tissue half-life, working locally by binding to a least 2 receptors: CYSLTR1 and CYSLTR2. CYSLTR1 antagonists (e.g. Montelukast and Zafirlukast) have been used for the management of AR, asthma and to a lesser extent nasal polyposis. Studies in CRS demonstrated levels of cysteinyl leukotrienes that were significantly higher in eosinophilic polyps compared to control tissue independent of atopy (1043). A later study confirmed and extended these findings demonstrating the highest levels of cysteinyl leukotrienes in aspirin sensitive polyps, followed by CRSwNP, CRSsNP and then normal mucosa (915, 1046). Corresponding increases were also seen in expression of the enzymes 5-LO and LTC4 synthase (1046).
The action of Cox-1 or Cox-2 enzymes results in the generation of prostanoids: prostaglandins, prostacyclin and thromboxane. Cox-1 is constitutively expressed while Cox-2 is inducible, the latter typically up-regulated in inflamed tissues, while the former can be influenced by topical glucocorticoid treatment (1047). Subsequent activity of the corresponding synthase enzymes produces PGD2 and PGE2 and from the perspective of airway disease, these are the most notable prostanoids (1042). PGD2 acts via binding to prostanoid receptors triggering proinflammatory effects including chemotaxis, de-granulation and enhanced survival of eosinophils (1048-1050) as well as migration of Th2 lymphocytes (1051). Increased PGD2 synthase enzyme levels were demonstrated in CRSwNP (1052) and differential PGD2 receptor expression was associated with polyposis (1053). PGE2 may be more significant from a clinical perspective, as it triggers bronchodilation acting via the EP2 prostanoid receptor (1054). In addition, PGE2 exhibits an array of primarily anti-inflammatory, protective effects by direct inhibition of leukotriene production (1055). Interestingly, PGE2 levels, cox-2 levels and PGE2 synthase levels are all decreased in nasal polyps (1010, 1046, 1052, 1056). Expression of the E-prostanoid receptors may also be altered in CRS (915). Studies have suggested that staphylococcal superantigenic toxins (SAg) may interact with the PGE2 pathway as well. SAg suppresses the PGE2 pathway while PGE2 blunts the proinflammatory effects of SAgs (605, 606). Most significantly, a recent study demonstrated that in contrast to normal fibroblasts, polyp fibroblasts fail to up-regulate the cox pathway in response to inflammatory stimuli (595).
Abnormalities of the eicosanoid pathway have been associated with CRSwNP, with an up-regulation of the pro-inflammatory leukotriene pathway and a down-regulation of the primarily anti-inflammatory PGE2 pathway
In summary, alterations in the eicosanoid pathways have been identified most prominently in CRSwNP, with an up-regulation of the pro-inflammatory leukotriene pathway and the downregulation of the primarily anti-inflammatory PGE2 pathway. It has been suggested that this pro-inflammatory environment, perhaps modified by colonized Staphylococcus aureus, may be central to the aetiology of nasal polyposis (594).
Eicosanoids are signaling molecules with immunologic and inflammatory properties generated by oxidative metabolism of arachidonic acid (AA) (1041, 1042). Disturbances in this pathway have been most closely associated with aspirin-sensitive nasal polyposis, but abnormalities have also been suggested to potentially underlie aspirin-tolerant CRSwNP as well. There are several families of classical eicosanoids with distinct properties: (25) leukotrienes (14), prostaglandins (PGD2, PGE2 and PGF2) (594); prostacyclin (PGI2); and thromboxane (TXA2) (625). Leukotrienes are generated by lipoxygenase (5-LO) activity, while the other 3 are generated by cyclooxygenase enzyme (Cox-1 and Cox-2) activity. Also relevant are the lipoxans, technically termed non-classical eicosanoids, which are generated by 12/15 lipoxygenase (12/15-LO) activity. In general, lipoxans and PGE2 have anti-inflammatory effects while the rest are all proinflammatory.
Leukotriene formation requires 5-LO activity that gives rise to the LTA4 precursor; subsequent enzyme activity results in the production of LTB4, LTC4, LTD4 and LTE4. The latter three are known as the cysteinyl leukotrienes, formerly termed slow reacting substance of anaphylaxis (SRSA), and require leukotriene C4 synthase activity (LTC4 synthase). Genetic polymorphisms in LTC4 synthase have been associated with CRSwNP in some studies (1043) and it has been suggested that this enzyme may be the engine of aspirin intolerance (1044, 1045). The primary sources of leukotrienes in the airway mucosa are mast cells and eosinophils, with effects including increased vascular permeability, vasodilation, leukocyte chemotaxis, broncho-constriction and mucus secretion. Leukotrienes have a short tissue half-life, working locally by binding to a least 2 receptors: CYSLTR1 and CYSLTR2. CYSLTR1 antagonists (e.g. Montelukast and Zafirlukast) have been used for the management of AR, asthma and to a lesser extent nasal polyposis. Studies in CRS demonstrated levels of cysteinyl leukotrienes that were significantly higher in eosinophilic polyps compared to control tissue independent of atopy (1043). A later study confirmed and extended these findings demonstrating the highest levels of cysteinyl leukotrienes in aspirin sensitive polyps, followed by CRSwNP, CRSsNP and then normal mucosa (915, 1046). Corresponding increases were also seen in expression of the enzymes 5-LO and LTC4 synthase (1046).
The action of Cox-1 or Cox-2 enzymes results in the generation of prostanoids: prostaglandins, prostacyclin and thromboxane. Cox-1 is constitutively expressed while Cox-2 is inducible, the latter typically up-regulated in inflamed tissues, while the former can be influenced by topical glucocorticoid treatment (1047). Subsequent activity of the corresponding synthase enzymes produces PGD2 and PGE2 and from the perspective of airway disease, these are the most notable prostanoids (1042). PGD2 acts via binding to prostanoid receptors triggering proinflammatory effects including chemotaxis, de-granulation and enhanced survival of eosinophils (1048-1050) as well as migration of Th2 lymphocytes (1051). Increased PGD2 synthase enzyme levels were demonstrated in CRSwNP (1052) and differential PGD2 receptor expression was associated with polyposis (1053). PGE2 may be more significant from a clinical perspective, as it triggers bronchodilation acting via the EP2 prostanoid receptor (1054). In addition, PGE2 exhibits an array of primarily anti-inflammatory, protective effects by direct inhibition of leukotriene production (1055). Interestingly, PGE2 levels, cox-2 levels and PGE2 synthase levels are all decreased in nasal polyps (1010, 1046, 1052, 1056). Expression of the E-prostanoid receptors may also be altered in CRS (915). Studies have suggested that staphylococcal superantigenic toxins (SAg) may interact with the PGE2 pathway as well. SAg suppresses the PGE2 pathway while PGE2 blunts the proinflammatory effects of SAgs (605, 606). Most significantly, a recent study demonstrated that in contrast to normal fibroblasts, polyp fibroblasts fail to up-regulate the cox pathway in response to inflammatory stimuli (595).
Abnormalities of the eicosanoid pathway have been associated with CRSwNP, with an up-regulation of the pro-inflammatory leukotriene pathway and a down-regulation of the primarily anti-inflammatory PGE2 pathway
In summary, alterations in the eicosanoid pathways have been identified most prominently in CRSwNP, with an up-regulation of the pro-inflammatory leukotriene pathway and the downregulation of the primarily anti-inflammatory PGE2 pathway. It has been suggested that this pro-inflammatory environment, perhaps modified by colonized Staphylococcus aureus, may be central to the aetiology of nasal polyposis (594).
4.2.5. Microarray studies
Microarray studies have been used on CRS tissues, primarily nasal polyps, in an effort to (a) understand the pathophysiology; (b) explore the mechanism of corticosteroid efficacy; and (c) serve as a platform to guide future investigations. The first study compared tissue from patients with AR vs. those with AR plus nasal polyps. Increased expression of the mammaglobulin gene was seen in nasal polyps, in comparison to patients with rhinitis alone; other genes associated with neoplastic growth were also up-regulated (1057). Another early study compared nasal polyps before and after oral glucocorticoid treatment. In this study, uteroglobulin- also known as CC10 -demonstrated the greatest increase while β-defensin showed the most marked down-regulation in response to corticosteroids (1058). Uteroglobulin has established diverse anti-inflammatory and immunomodulatory properties. Microarray techniques have also been utilized to directly compare nasal polyps to normal control tissues. Relative to normal tissue, the most up-regulated genes in polyps included statherin, prolactin-induced protein (PIP), lactoferrin and deleted in malignant brain tumor 1 (DMBT1), while the most down-regulated gene was uteroglobulin/CC10 (1059). The polyp patients were separated into 2 groups: oedematous polyps which were highly eosinophilic and glandular polyps which were less eosinophilic. Immunohistochemical studies indicated that lactoferrin, DMBTI and PIP were increased in the glands of only the 'glandular-type' polyps, not the oedematous polyps. CC10/uteroglobulin was present primarily in the epithelium of normal controls and greatly decreased in both forms of polyposis. Interestingly, this study did not see significant changes in expression of many of the genes commonly associated with CRSwNP including IL-4, IL-5 and GM-CSF (3). Expanded microarrays and bio-informatic analyses were used in a larger study comparing 3 groups of patients (10 in each group): normal controls, aspirin-sensitive polyps (ASA) and aspirin-tolerant polyps (CRSwNP) (1029). This study demonstrated substantial agreement between the 2 polyp phenotypes but increased expression of periostin and met protoncogene and decreased expression of PIP was seen relative to control tissues. Periostin is a protein that is highly expressed in the airway epithelium of asthma patients believed to play a role in TGF-β activation, collagen deposition, fibrosis and remodeling (1060). The Met gene (or c-Met) encodes hepatocyte growth factor receptor (HGFR), an epithelial membrane receptor known to bind hepatocyte growth factor (HGF). HGF and HGFR expression are increased in CRSwNP (1061). From a functional perspective, this ligand-receptor binding is believed to play a role in wound healing and inflammation in lower airway epithelium. In the human upper airway, preliminary evidence suggests that genetic variation in the c-Met pathway is associated with CRSwNP (1062), perhaps through the loss of HGF mediated down regulation of the effects of Th2 cytokines (1063). Pip protein, whose expression is decreased in the polyp phenotypes, has immunologic and water transport functions but no clear pathophysiologic role in CRS.
Smaller studies, analyzing more narrow phenotypes have also utilized microarray technology. Anand et al., analyzed tissue from non-allergic, CRSsNP patients compared to controls and demonstrated increased expression of IL-6, IL-12A, IL-13 and TNF-α in disease (1064). Wang et al., compared Asian polyps with normal tissue and noted increased expression of IL-17 and IL-17R in the CRSwNP patients (1065). Lee et al., compared polyps and control mucosa, with results that generally concurred with the findings of Stankovic et al. (1066). Orlandi et al., compared classic AFS patients and eosinophilic mucin rhinosinusitis patients (EMRS) (730). The 2 groups differed only in the ability to identify fungi by routine histology or culture and gene expression profiles demonstrated marked similarities to each other as opposed to dramatic differences to controls. Figueiredo et al., compared polyp tissue and surrounding non-polypoid tissue from non-atopic patients, with control tissues. Results indicated increased IL-5 expression in the polyps and increased TGF-β expression in the adjacent inflamed mucosa (1067). A study by Bolger et al., demonstrated that systemic glucocorticoids decreased expression of several chemokine and leukotriene receptor genes (1068). A small study by Payne et al., focused on non-eosinphilic polyps, demonstrating significant down regulation of IL-4, IL-13 and IFN-γ with up-regulation of IL-6, IL-8, SCF (Stem Cell Factor) and hypoxia inducible factor 1α (HIF-1α) in polyps versus control tissues (1017).These results were interpreted to suggest that NE polyps have a distinct pathogenesis. A later study by Wu et al. comparing atopic Asian polyps to both normal tissue and AR tissue revealed significantly increased expression of CCL20 in the polyp cohort (1069). Lastly, a very recent study comparing nasal polyps and control tissue demonstrated significantly increased expression of IL-8, MMP10, NOS2A and ALOX15 in polyps; decreased expression of ALOX12, LTF and DMBT1 was also seen (1070).
The results of these studies reveal substantial differences, despite apparent similarities in clinical phenotypes in many cases. As a specific example comparing two of the largest studies, results from Liu et al.(1059), demonstrated that statherin, PIP, lactoferrin and DMBT1 were elevated while uteroglobulin was decreased. In contrast, Stankovic et al.(1029), reported that statherin, PIP, lactoferrin and DMBT1 were decreased and uteroglobulin was unchanged. Variations in patient selection, experimental technique, sample size and pre-operative treatment account, at least in part, for differences. Despite enormous promise, the application of microarray technology has thus far failed to result in any major breakthrough in our understanding of CRS.
Microarray studies have been used on CRS tissues, primarily nasal polyps, in an effort to (a) understand the pathophysiology; (b) explore the mechanism of corticosteroid efficacy; and (c) serve as a platform to guide future investigations. The first study compared tissue from patients with AR vs. those with AR plus nasal polyps. Increased expression of the mammaglobulin gene was seen in nasal polyps, in comparison to patients with rhinitis alone; other genes associated with neoplastic growth were also up-regulated (1057). Another early study compared nasal polyps before and after oral glucocorticoid treatment. In this study, uteroglobulin- also known as CC10 -demonstrated the greatest increase while β-defensin showed the most marked down-regulation in response to corticosteroids (1058). Uteroglobulin has established diverse anti-inflammatory and immunomodulatory properties. Microarray techniques have also been utilized to directly compare nasal polyps to normal control tissues. Relative to normal tissue, the most up-regulated genes in polyps included statherin, prolactin-induced protein (PIP), lactoferrin and deleted in malignant brain tumor 1 (DMBT1), while the most down-regulated gene was uteroglobulin/CC10 (1059). The polyp patients were separated into 2 groups: oedematous polyps which were highly eosinophilic and glandular polyps which were less eosinophilic. Immunohistochemical studies indicated that lactoferrin, DMBTI and PIP were increased in the glands of only the 'glandular-type' polyps, not the oedematous polyps. CC10/uteroglobulin was present primarily in the epithelium of normal controls and greatly decreased in both forms of polyposis. Interestingly, this study did not see significant changes in expression of many of the genes commonly associated with CRSwNP including IL-4, IL-5 and GM-CSF (3). Expanded microarrays and bio-informatic analyses were used in a larger study comparing 3 groups of patients (10 in each group): normal controls, aspirin-sensitive polyps (ASA) and aspirin-tolerant polyps (CRSwNP) (1029). This study demonstrated substantial agreement between the 2 polyp phenotypes but increased expression of periostin and met protoncogene and decreased expression of PIP was seen relative to control tissues. Periostin is a protein that is highly expressed in the airway epithelium of asthma patients believed to play a role in TGF-β activation, collagen deposition, fibrosis and remodeling (1060). The Met gene (or c-Met) encodes hepatocyte growth factor receptor (HGFR), an epithelial membrane receptor known to bind hepatocyte growth factor (HGF). HGF and HGFR expression are increased in CRSwNP (1061). From a functional perspective, this ligand-receptor binding is believed to play a role in wound healing and inflammation in lower airway epithelium. In the human upper airway, preliminary evidence suggests that genetic variation in the c-Met pathway is associated with CRSwNP (1062), perhaps through the loss of HGF mediated down regulation of the effects of Th2 cytokines (1063). Pip protein, whose expression is decreased in the polyp phenotypes, has immunologic and water transport functions but no clear pathophysiologic role in CRS.
Smaller studies, analyzing more narrow phenotypes have also utilized microarray technology. Anand et al., analyzed tissue from non-allergic, CRSsNP patients compared to controls and demonstrated increased expression of IL-6, IL-12A, IL-13 and TNF-α in disease (1064). Wang et al., compared Asian polyps with normal tissue and noted increased expression of IL-17 and IL-17R in the CRSwNP patients (1065). Lee et al., compared polyps and control mucosa, with results that generally concurred with the findings of Stankovic et al. (1066). Orlandi et al., compared classic AFS patients and eosinophilic mucin rhinosinusitis patients (EMRS) (730). The 2 groups differed only in the ability to identify fungi by routine histology or culture and gene expression profiles demonstrated marked similarities to each other as opposed to dramatic differences to controls. Figueiredo et al., compared polyp tissue and surrounding non-polypoid tissue from non-atopic patients, with control tissues. Results indicated increased IL-5 expression in the polyps and increased TGF-β expression in the adjacent inflamed mucosa (1067). A study by Bolger et al., demonstrated that systemic glucocorticoids decreased expression of several chemokine and leukotriene receptor genes (1068). A small study by Payne et al., focused on non-eosinphilic polyps, demonstrating significant down regulation of IL-4, IL-13 and IFN-γ with up-regulation of IL-6, IL-8, SCF (Stem Cell Factor) and hypoxia inducible factor 1α (HIF-1α) in polyps versus control tissues (1017).These results were interpreted to suggest that NE polyps have a distinct pathogenesis. A later study by Wu et al. comparing atopic Asian polyps to both normal tissue and AR tissue revealed significantly increased expression of CCL20 in the polyp cohort (1069). Lastly, a very recent study comparing nasal polyps and control tissue demonstrated significantly increased expression of IL-8, MMP10, NOS2A and ALOX15 in polyps; decreased expression of ALOX12, LTF and DMBT1 was also seen (1070).
The results of these studies reveal substantial differences, despite apparent similarities in clinical phenotypes in many cases. As a specific example comparing two of the largest studies, results from Liu et al.(1059), demonstrated that statherin, PIP, lactoferrin and DMBT1 were elevated while uteroglobulin was decreased. In contrast, Stankovic et al.(1029), reported that statherin, PIP, lactoferrin and DMBT1 were decreased and uteroglobulin was unchanged. Variations in patient selection, experimental technique, sample size and pre-operative treatment account, at least in part, for differences. Despite enormous promise, the application of microarray technology has thus far failed to result in any major breakthrough in our understanding of CRS.
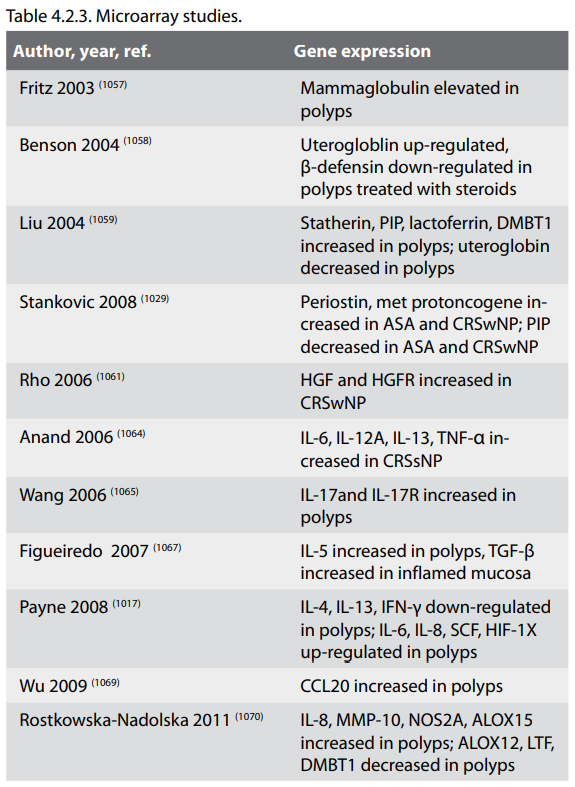
4.3. Diagnosis
4.3.1. Summary
A range of diagnostic tests is available to validate the clinical symptoms and signs of rhinosinusitis. However, for the majority of patients, the diagnosis is made in primary care based on symptoms alone. Objective investigations exist to corroborate the diagnosis, notably endoscopy and CT scanning which can be semi-quantitatively scored to assist in the stratification of disease and its response to therapy. Additional tests may help in the differential diagnosis of aetiological and predisposing factors but some remain the preserve of tertiary research facilities.
4.3.2. Assessment of rhinosinusitis symptoms
4.3.2.1. Symptoms of rhinosinusitis
Subjective assessment of rhinosinusitis is based on symptoms:
The symptoms are principally the same in acute (ARS) and chronic rhinosinusitis with and without nasal polyposis (CRSw/ sNP), but the symptom pattern and intensity may vary. Acute forms of infections have usually more distinct and often more severe symptoms.
4.3.3. Diagnosis of ARS
Acute rhinosinusitis in adults is defined as a sudden onset of two or more symptoms, one of which should be either nasal blockage/obstruction/congestion or nasal discharge (anterior/ posterior nasal drip):
± facial pain/pressure,
± reduction or loss of smell
for <12 weeks;
This may be supported by endoscopic signs of purulent discharge from the middle meatus, oedema/ mucosal obstruction primarily in the middle meatus Imaging is rarely performed except in severe/complicated cases.
4.3.1. Summary
A range of diagnostic tests is available to validate the clinical symptoms and signs of rhinosinusitis. However, for the majority of patients, the diagnosis is made in primary care based on symptoms alone. Objective investigations exist to corroborate the diagnosis, notably endoscopy and CT scanning which can be semi-quantitatively scored to assist in the stratification of disease and its response to therapy. Additional tests may help in the differential diagnosis of aetiological and predisposing factors but some remain the preserve of tertiary research facilities.
4.3.2. Assessment of rhinosinusitis symptoms
4.3.2.1. Symptoms of rhinosinusitis
Subjective assessment of rhinosinusitis is based on symptoms:
- nasal blockage, congestion or stuffiness;
-
nasal discharge or postnasal drip, often mucopurulent;
-
facial pain or pressure, headache, and
-
reduction/loss of smell.
The symptoms are principally the same in acute (ARS) and chronic rhinosinusitis with and without nasal polyposis (CRSw/ sNP), but the symptom pattern and intensity may vary. Acute forms of infections have usually more distinct and often more severe symptoms.
4.3.3. Diagnosis of ARS
Acute rhinosinusitis in adults is defined as a sudden onset of two or more symptoms, one of which should be either nasal blockage/obstruction/congestion or nasal discharge (anterior/ posterior nasal drip):
± facial pain/pressure,
± reduction or loss of smell
for <12 weeks;
This may be supported by endoscopic signs of purulent discharge from the middle meatus, oedema/ mucosal obstruction primarily in the middle meatus Imaging is rarely performed except in severe/complicated cases.
4.3.4. Diagnosis of CRS
Chronic rhinosinusitis, with or without nasal polyps in adults is defined as:
This should be supported by demonstrable disease Either endoscopic signs of:
Using this symptomatic definition (8), the GA2LEN study has demonstrated significant variation in the prevalence of self-reported CRS across Europe, with a mean of 10.9% of participants, but a range of 6.9% (Brandenburg, Helsinki) to 27.1 (Coimbra) (12). As a percentage of EP3OS-defined CRS patients, the prevalence of component symptoms of CRS was 83.7% blocked nose, 63.6% nasal discharge, 64.7% pain or pressure, and 48.5% reduced sense of smell.
It is appropriate that the definition is symptom based, as it is this that drives patients to seek medical care for their CRS. However, the presence of supporting findings is important to exclude differential diagnoses. A recent study of 125 patients with CRS based on symptoms found 40% had no radiological evidence of disease on CT scan (1072). In a subset of the GA2LEN study (11), 61.7% of symptom-positive subjects had a positive endoscopy, while 38.0% of symptom negative subjects had a positive endoscopy. Symptom-based CRS was significantly associated with a positive endoscopy (OR 2.62: 95% CI 1.57 – 4.39, p<0.001).
Symptoms remain the mainstay of diagnosis in primary care
In a group of patients meeting the 1997 Rhinosinusitis Task Force (RSTF) definition (523) of chronic rhinosinusitis (≥3 symptoms from a defined list, with severity rating of>5/10) were subjected to same-day endoscopy and CT scanning, seventeen (22%) of 78 patients had positive endoscopic and CT results (1073). There were 20 (26%) of 78 patients with negative endoscopic and positive CT results. Six (8%) patients had positive endoscopic and negative CT results, and 35 (45%) had negative endoscopic and negative CT results. Thus, only 55% of symptom-positive CRS had positive supporting findings. The lower rates of positive endoscopy in this series may reflect the less strict symptom criteria used in the study, including 'minor' symptoms such as headache, fatigue and cough within the definition. The sensitivity of endoscopy was rather low (46%), but the positive and negative predictive values indicating the proportion of patients with and without disease was better, at 74% and 64% respectively
Since this study, new guidelines have been issued by the AAOHNS, with diagnostic criteria broadly in line with the EP3 OS criteria above. Bhattacharya et al repeated the validation study in a group of 202 patients, of which 178 met the symptomatic criteria. Of the symptom positive group 50.6% had neither positive changes on CT nor positi7ve endoscopy, while of the symptom negative group, 45.8% had positive CT or endoscopy (1074). Therefore, using the findings of disease on either CT or endoscopy as the 'gold standard', symptoms alone have a sensitivity of 89%, specificity of 12%, PPV of 49% and NPV of 54%. It is notable that 31% of patients failing to meet symptom or endoscopic criteria had positive CT scans (LM≥4).
Chronic rhinosinusitis, with or without nasal polyps in adults is defined as:
- inflammation of the nose and the paranasal sinuses characterised by two or more symptoms, one of which should be either nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip):
-
± facial pain/pressure
-
± reduction or loss of smell
This should be supported by demonstrable disease Either endoscopic signs of:
- nasal polyps, and/or
-
mucopurulent discharge primarily from middle meatus
and/or
-
oedema/mucosal obstruction primarily in middle
meatus
- CT changes:
-
mucosal changes within the ostiomeatal complex and/or
sinuses
Using this symptomatic definition (8), the GA2LEN study has demonstrated significant variation in the prevalence of self-reported CRS across Europe, with a mean of 10.9% of participants, but a range of 6.9% (Brandenburg, Helsinki) to 27.1 (Coimbra) (12). As a percentage of EP3OS-defined CRS patients, the prevalence of component symptoms of CRS was 83.7% blocked nose, 63.6% nasal discharge, 64.7% pain or pressure, and 48.5% reduced sense of smell.
It is appropriate that the definition is symptom based, as it is this that drives patients to seek medical care for their CRS. However, the presence of supporting findings is important to exclude differential diagnoses. A recent study of 125 patients with CRS based on symptoms found 40% had no radiological evidence of disease on CT scan (1072). In a subset of the GA2LEN study (11), 61.7% of symptom-positive subjects had a positive endoscopy, while 38.0% of symptom negative subjects had a positive endoscopy. Symptom-based CRS was significantly associated with a positive endoscopy (OR 2.62: 95% CI 1.57 – 4.39, p<0.001).
Symptoms remain the mainstay of diagnosis in primary care
In a group of patients meeting the 1997 Rhinosinusitis Task Force (RSTF) definition (523) of chronic rhinosinusitis (≥3 symptoms from a defined list, with severity rating of>5/10) were subjected to same-day endoscopy and CT scanning, seventeen (22%) of 78 patients had positive endoscopic and CT results (1073). There were 20 (26%) of 78 patients with negative endoscopic and positive CT results. Six (8%) patients had positive endoscopic and negative CT results, and 35 (45%) had negative endoscopic and negative CT results. Thus, only 55% of symptom-positive CRS had positive supporting findings. The lower rates of positive endoscopy in this series may reflect the less strict symptom criteria used in the study, including 'minor' symptoms such as headache, fatigue and cough within the definition. The sensitivity of endoscopy was rather low (46%), but the positive and negative predictive values indicating the proportion of patients with and without disease was better, at 74% and 64% respectively
Since this study, new guidelines have been issued by the AAOHNS, with diagnostic criteria broadly in line with the EP3 OS criteria above. Bhattacharya et al repeated the validation study in a group of 202 patients, of which 178 met the symptomatic criteria. Of the symptom positive group 50.6% had neither positive changes on CT nor positi7ve endoscopy, while of the symptom negative group, 45.8% had positive CT or endoscopy (1074). Therefore, using the findings of disease on either CT or endoscopy as the 'gold standard', symptoms alone have a sensitivity of 89%, specificity of 12%, PPV of 49% and NPV of 54%. It is notable that 31% of patients failing to meet symptom or endoscopic criteria had positive CT scans (LM≥4).
4.3.5. Symptoms reported in CRS
An overlap of symptoms with ARS, those of chronic rhinosinusitis are typically of lesser intensity. In addition to the diagnostic symptoms listed above, there are several minor symptoms including ear pain or pressure, dizziness, halitosis, dental pain, distant and general symptoms including nasal, pharyngeal, laryngeal and tracheal irritation, dysphonia and cough, drowsiness, malaise and sleep disturbance, presenting in numerous combinations (235, 239). . There is a surprising paucity of epidemiological studies reporting symptoms in CRS. Most studies utilise a questionnaire asking patients to rate the severity of specific symptoms, thus encouraging patients to report only on those listed, and to report symptoms that they might not have done so if asked to provide a list of symptoms without guidance. Consequently, different patterns are reported in the published literature, depending on the questionnaire utilised in the study. For example, in a study using the 'Cologne questionnaire', the most commonly reported symptoms of CRS were nasal obstruction (92%), postnasal drip (87%) and 'dry upper respiratory tract syndrome' (68%) (239). Another study asking patients to rate the severity of symptoms included in the RSTF diagnostic criteria reports nasal obstruction (84%), postnasal drip (82%), and facial congestion (79%) as the most prevalent (1075).
Nasal obstruction is one of the most commonly reported symptoms of CRS. It consists of 3 main components; congestion due to dilation of the venous sinusoids as a result of inflammation and oedema, nasal fibrosis and nasal polyposis, and may only be partly reversible by topical decongestant.
Nasal discharge may be anterior or posterior, and may vary greatly in composition. Patients may report profuse watery discharge or thick purulent secretions. Facial pain is perhaps one of the most variable symptoms, with reported prevalence in patients with CRS ranging from 18 % (1076) – 77.9% (1075). In a large longitudinal study, diagnosis of CRS is associated with a ninefold increased risk of reporting chronic headache compared with the general population, and symptoms were significantly improved after treatment with nasal surgery and nasal corticosteroids (1077). Facial pain and it's differential diagnosis is discussed in more detail in section 4d. Olfactory disturbance is common, due to physical prevention of odorants reaching the olfactory cleft, and oedema in this area. A recent population-based epidemiological study found that a history of nasal polyps was a significant risk factor for olfactory impairment (OR = 2.33, 95% CI, 1.13–4.59) (1078). In a study of 367 patients (1079) with a diagnosis of CRS, the presence of polyposis was associated with significantly increased risk of hyposmia (OR 2.4, 95% CI 1.3-4.2, P = 0.003) and anosmia (OR 13.2, 95% CI 5.7-30.7, P < 0.001) compared with non-polyp CRS. The pathophysiology behind these and other symptoms found in CRS is discussed elsewhere (75).
Sleep impairment is a significant problem for patients with inflammatory disorders of the upper respiratory tract, such as CRSsNP and CRSwNP. Nasal congestion is associated with sleep-disordered breathing and is thought to be a key cause of sleep impairment. Poor sleep can lead to fatigue, daytime somnolence, impaired daytime functioning as reflected in lower levels of productivity at work or school, and a reduced quality of life (483, 1080, 1081). Treatment with intranasal corticosteroids has been shown to reduce nasal congestion in inflammatory disorders of the upper respiratory tract. There is a growing amount of evidence that a reduction in congestion with intranasal corticosteroids is associated with improved sleep, reduced daytime sleepiness, and enhanced patient quality of life (1082).
Serrano et al. (547) showed in a population-based, cross-sectional, case-control study that NP patients have a two-fold higher risk of suffering sleep disturbance. A quarter of NP patients (24.6 per cent) reported a feeling of general discomfort due to their nasal condition, during the day as well as the night in most of these cases (61.2 per cent).
An overlap of symptoms with ARS, those of chronic rhinosinusitis are typically of lesser intensity. In addition to the diagnostic symptoms listed above, there are several minor symptoms including ear pain or pressure, dizziness, halitosis, dental pain, distant and general symptoms including nasal, pharyngeal, laryngeal and tracheal irritation, dysphonia and cough, drowsiness, malaise and sleep disturbance, presenting in numerous combinations (235, 239). . There is a surprising paucity of epidemiological studies reporting symptoms in CRS. Most studies utilise a questionnaire asking patients to rate the severity of specific symptoms, thus encouraging patients to report only on those listed, and to report symptoms that they might not have done so if asked to provide a list of symptoms without guidance. Consequently, different patterns are reported in the published literature, depending on the questionnaire utilised in the study. For example, in a study using the 'Cologne questionnaire', the most commonly reported symptoms of CRS were nasal obstruction (92%), postnasal drip (87%) and 'dry upper respiratory tract syndrome' (68%) (239). Another study asking patients to rate the severity of symptoms included in the RSTF diagnostic criteria reports nasal obstruction (84%), postnasal drip (82%), and facial congestion (79%) as the most prevalent (1075).
Nasal obstruction is one of the most commonly reported symptoms of CRS. It consists of 3 main components; congestion due to dilation of the venous sinusoids as a result of inflammation and oedema, nasal fibrosis and nasal polyposis, and may only be partly reversible by topical decongestant.
Nasal discharge may be anterior or posterior, and may vary greatly in composition. Patients may report profuse watery discharge or thick purulent secretions. Facial pain is perhaps one of the most variable symptoms, with reported prevalence in patients with CRS ranging from 18 % (1076) – 77.9% (1075). In a large longitudinal study, diagnosis of CRS is associated with a ninefold increased risk of reporting chronic headache compared with the general population, and symptoms were significantly improved after treatment with nasal surgery and nasal corticosteroids (1077). Facial pain and it's differential diagnosis is discussed in more detail in section 4d. Olfactory disturbance is common, due to physical prevention of odorants reaching the olfactory cleft, and oedema in this area. A recent population-based epidemiological study found that a history of nasal polyps was a significant risk factor for olfactory impairment (OR = 2.33, 95% CI, 1.13–4.59) (1078). In a study of 367 patients (1079) with a diagnosis of CRS, the presence of polyposis was associated with significantly increased risk of hyposmia (OR 2.4, 95% CI 1.3-4.2, P = 0.003) and anosmia (OR 13.2, 95% CI 5.7-30.7, P < 0.001) compared with non-polyp CRS. The pathophysiology behind these and other symptoms found in CRS is discussed elsewhere (75).
Sleep impairment is a significant problem for patients with inflammatory disorders of the upper respiratory tract, such as CRSsNP and CRSwNP. Nasal congestion is associated with sleep-disordered breathing and is thought to be a key cause of sleep impairment. Poor sleep can lead to fatigue, daytime somnolence, impaired daytime functioning as reflected in lower levels of productivity at work or school, and a reduced quality of life (483, 1080, 1081). Treatment with intranasal corticosteroids has been shown to reduce nasal congestion in inflammatory disorders of the upper respiratory tract. There is a growing amount of evidence that a reduction in congestion with intranasal corticosteroids is associated with improved sleep, reduced daytime sleepiness, and enhanced patient quality of life (1082).
Serrano et al. (547) showed in a population-based, cross-sectional, case-control study that NP patients have a two-fold higher risk of suffering sleep disturbance. A quarter of NP patients (24.6 per cent) reported a feeling of general discomfort due to their nasal condition, during the day as well as the night in most of these cases (61.2 per cent).
4.3.6. Assessment of symptom severity
The severity of the overall symptoms of CRS can be estimated using many different grading tools.
Both the strength or degree and duration of symptoms should be assessed. The duration of the symptoms is evaluated as symptomatic or symptom-free moments in given time periods, i.e. as hours during the recording period or as day per week. "No symptom" can be regarded as a consistent finding in most studies.
A validation study has shown 'mild disease' to be defined as a VAS score of 0-3 inclusive, moderate as >3-7 inclusive, and severe as ≥7. In general, overall quality of life is more likely to be affected with scores of 5 or more (1083).
In addition the severity of individual symptoms can be measured, including different aspects of quality of life. This is done using validated questionnaires, described below.
Endoscopy and CT scanning corroborate the clinical symptoms and signs
The severity of the overall symptoms of CRS can be estimated using many different grading tools.
- recorded as such: no symptom, mild, moderate or severe
-
recorded as numbers: from 0 to 5 or as many degrees as
needed;
-
recorded as VAS score on a line giving a measurable
continuum (0 – 10 cm).
Both the strength or degree and duration of symptoms should be assessed. The duration of the symptoms is evaluated as symptomatic or symptom-free moments in given time periods, i.e. as hours during the recording period or as day per week. "No symptom" can be regarded as a consistent finding in most studies.
A validation study has shown 'mild disease' to be defined as a VAS score of 0-3 inclusive, moderate as >3-7 inclusive, and severe as ≥7. In general, overall quality of life is more likely to be affected with scores of 5 or more (1083).
In addition the severity of individual symptoms can be measured, including different aspects of quality of life. This is done using validated questionnaires, described below.
Endoscopy and CT scanning corroborate the clinical symptoms and signs
4.3.7. Examination
4.3.7.1. Anterior rhinoscopy
Anterior rhinoscopy alone is of limited value, but nonetheless, remains the first step in examining a patient with these diseases.
4.3.7.2 Nasal Endoscopy
This may be performed without and with decongestion and semi-quantitative scores for polyps, oedema, discharge, crusting and scarring (post-operatively) can be obtained at baseline and at regular intervals following therapeutic interventions eg at 3, 6, 9 and 12 months (5) (table 4.3.1). Nasal endoscopy affords significantly better illumination and visualization compared to anterior rhinoscopy for examination of the middle and superior meati as well as the nasopharynx and mucociliary drainage pathways. Bhattacharyya et al. confirmed the added utility of nasal endoscopy in the diagnosis of chronic rhinosinusits (1074)..However, in post surgical CRS patients, nasal endoscopy does not necessarily correlate with symptoms (1084).
4.3.7.3. Nasal cytology, biopsy and bacteriology
Generally cytology has not proved a useful tool in diagnosis of rhinosinusitis although a formal biopsy may be indicated to exclude more sinister and severe conditions such as neoplasia and the vasculitides. Techniques include lavage with 0.9% saline, microsuction, nasal brushes, disposable scrapers with a cupped end or small mucosal samples taken with Gerritsma forceps. These are largely used for clinical research. However, a correlation has been shown between the cellular content obtained by middle meatal and broncho-alveolar lavage in patients with CRS and asthma (1086).
Swabs, aspirates, lavages and biopsies may also be used to obtain microbiological samples. Several microbiology studies (263-267) (Evidence Level IIb) have shown a reasonable correlation between specimens taken from the middle meatus under endoscopic control and proof puncture of the maxillary sinus or swabs from the ethmoid taken per-operatively leading to the possibility of microbiological confirmation of both the pathogen and its response to therapy (Table 4.3.2). A meta-analysis showed anccuracy of 87% with a lower end confidence level of 81.3% for the endoscopically directed middle meatal culture when compared with maxillary sinus taps in acute maxillary sinus infection (248).
More sophisticated techniques exist for the detection and identification of bacteria including immunohistochemistry and the detection and amplification of microbial RNA and DNA. Fluorescent in situ hybridization (FISH) and confocal microscopy are utilised to demonstrate bacteria in biofilms (582).
4.3.7.4. Sinus Transillumination
This technique was advocated in the 1970's as an inexpensive and efficacious screening modality for sinus pathology. However, the insensitivity and unspecificity makes it unreliable for the diagnosis of rhinosinusitis (1). More recently with the introduction of balloon sinuplastly, transillumination has been used for confirmation of proper placement of guide wires.
4.3.7.5. Imaging
The plain sinus x-ray, despite low cost and availability has limited usefulness for the diagnosis of rhinosinusitis due to underestimation of bony and soft tissue pathology compared to computed tomography (CT) and magnetic resonance imaging (MRI).
CT scanning is the modality of choice for the paranasal sinuses due to optimal display of air bone and soft tissue. However, it should not be regarded as the primary step in the diagnosis of the condition, except where there are unilateral signs and symptoms or other sinister signs, but rather corroborates history and endoscopic examination after failure of medical therapy. Much attention has recently been given to the radiation exposure associated with CT scans, the use of which have increased 20 fold in the last 30 years (1088, 1089). Thus several protocols have been developed to decrease radiation exposure with comparable or improved resolution (1090, 1091). Cone beam technology is becoming increasingly available and is associated with lower radiation exposure than conventional imaging. A study comparing cone beam CT (CBCT) with multislice CT (MSCT) for the sinuses in an anthropomorphic phantom model showed the effective dose of CBCT was 30uSv as compared with 200uSv and 1400uSv for low dose and standard protocols using MSCT (1092). MRI does not have the radiation risk and has improved soft tissue definition over CT scan with an ability to differentiate between soft tissue masses and retained/obstructed secretions. Thus, MRI compliments CT in the workup of suspected neoplastic processes. Comparison of staging accuracy of sinonasal disease between CT and MRI demonstrates close correlation between the two modalities (1093).
It should be noted that incidental abnormalities are found on scanning in up to a fifth of the 'normal' population (1). Thus, in the absence of symptoms, diagnosis of CRS based on radiology alone is inappropriate
A range of staging systems based on CT scanning have been described but the most commonly used is the Lund-Mackay system which is based on localization with points given for degree of opacification: 0 = normal, 1 = partial opacification, 2 = total opacification. These points are then applied to the maxillary, anterior ethmoid, posterior ethmoid, sphenoid, frontal sinus on each side. The osteomeatal complex is graded as 0 = not occluded, or 2 = occluded deriving a maximum score of 12 per side (1094).This scoring system has been validated in several studies (1095, 1096).
4.3.7.1. Anterior rhinoscopy
Anterior rhinoscopy alone is of limited value, but nonetheless, remains the first step in examining a patient with these diseases.
4.3.7.2 Nasal Endoscopy
This may be performed without and with decongestion and semi-quantitative scores for polyps, oedema, discharge, crusting and scarring (post-operatively) can be obtained at baseline and at regular intervals following therapeutic interventions eg at 3, 6, 9 and 12 months (5) (table 4.3.1). Nasal endoscopy affords significantly better illumination and visualization compared to anterior rhinoscopy for examination of the middle and superior meati as well as the nasopharynx and mucociliary drainage pathways. Bhattacharyya et al. confirmed the added utility of nasal endoscopy in the diagnosis of chronic rhinosinusits (1074)..However, in post surgical CRS patients, nasal endoscopy does not necessarily correlate with symptoms (1084).
4.3.7.3. Nasal cytology, biopsy and bacteriology
Generally cytology has not proved a useful tool in diagnosis of rhinosinusitis although a formal biopsy may be indicated to exclude more sinister and severe conditions such as neoplasia and the vasculitides. Techniques include lavage with 0.9% saline, microsuction, nasal brushes, disposable scrapers with a cupped end or small mucosal samples taken with Gerritsma forceps. These are largely used for clinical research. However, a correlation has been shown between the cellular content obtained by middle meatal and broncho-alveolar lavage in patients with CRS and asthma (1086).
Swabs, aspirates, lavages and biopsies may also be used to obtain microbiological samples. Several microbiology studies (263-267) (Evidence Level IIb) have shown a reasonable correlation between specimens taken from the middle meatus under endoscopic control and proof puncture of the maxillary sinus or swabs from the ethmoid taken per-operatively leading to the possibility of microbiological confirmation of both the pathogen and its response to therapy (Table 4.3.2). A meta-analysis showed anccuracy of 87% with a lower end confidence level of 81.3% for the endoscopically directed middle meatal culture when compared with maxillary sinus taps in acute maxillary sinus infection (248).
More sophisticated techniques exist for the detection and identification of bacteria including immunohistochemistry and the detection and amplification of microbial RNA and DNA. Fluorescent in situ hybridization (FISH) and confocal microscopy are utilised to demonstrate bacteria in biofilms (582).
4.3.7.4. Sinus Transillumination
This technique was advocated in the 1970's as an inexpensive and efficacious screening modality for sinus pathology. However, the insensitivity and unspecificity makes it unreliable for the diagnosis of rhinosinusitis (1). More recently with the introduction of balloon sinuplastly, transillumination has been used for confirmation of proper placement of guide wires.
4.3.7.5. Imaging
The plain sinus x-ray, despite low cost and availability has limited usefulness for the diagnosis of rhinosinusitis due to underestimation of bony and soft tissue pathology compared to computed tomography (CT) and magnetic resonance imaging (MRI).
CT scanning is the modality of choice for the paranasal sinuses due to optimal display of air bone and soft tissue. However, it should not be regarded as the primary step in the diagnosis of the condition, except where there are unilateral signs and symptoms or other sinister signs, but rather corroborates history and endoscopic examination after failure of medical therapy. Much attention has recently been given to the radiation exposure associated with CT scans, the use of which have increased 20 fold in the last 30 years (1088, 1089). Thus several protocols have been developed to decrease radiation exposure with comparable or improved resolution (1090, 1091). Cone beam technology is becoming increasingly available and is associated with lower radiation exposure than conventional imaging. A study comparing cone beam CT (CBCT) with multislice CT (MSCT) for the sinuses in an anthropomorphic phantom model showed the effective dose of CBCT was 30uSv as compared with 200uSv and 1400uSv for low dose and standard protocols using MSCT (1092). MRI does not have the radiation risk and has improved soft tissue definition over CT scan with an ability to differentiate between soft tissue masses and retained/obstructed secretions. Thus, MRI compliments CT in the workup of suspected neoplastic processes. Comparison of staging accuracy of sinonasal disease between CT and MRI demonstrates close correlation between the two modalities (1093).
It should be noted that incidental abnormalities are found on scanning in up to a fifth of the 'normal' population (1). Thus, in the absence of symptoms, diagnosis of CRS based on radiology alone is inappropriate
A range of staging systems based on CT scanning have been described but the most commonly used is the Lund-Mackay system which is based on localization with points given for degree of opacification: 0 = normal, 1 = partial opacification, 2 = total opacification. These points are then applied to the maxillary, anterior ethmoid, posterior ethmoid, sphenoid, frontal sinus on each side. The osteomeatal complex is graded as 0 = not occluded, or 2 = occluded deriving a maximum score of 12 per side (1094).This scoring system has been validated in several studies (1095, 1096).
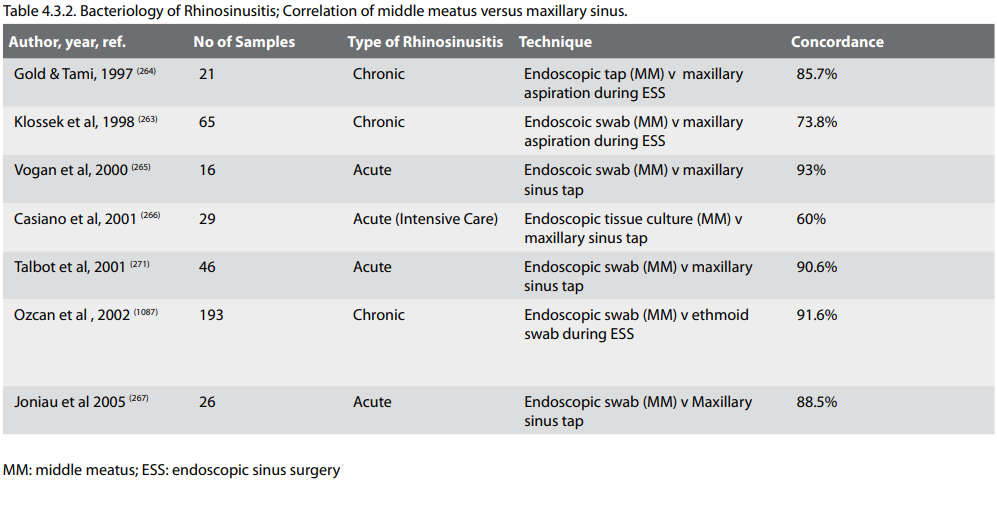
4.3.8. Additional assessment tools
A wide range of other diagnostic tests are available to assist with the differential diagnosis and to define predisposing and aetiological factors but many are only available in research departments
4.3.8.1. Mucociliary function
4.3.8.1.1. Nasomucociliary clearance
The use of saccharin, dye or radioactive particles to measure mucociliary transit time has been available for nearly thirty years (1097-1099). It allows one to recognize early alterations of sinosinusal homeostasis. Although a crude measure, it has the advantage of considering the entire mucociliary system and is useful if normal (< 35 minutes). However, if it is prolonged, it does not distinguish between primary or secondary causes of ciliary dysfunction. Nasomucociliary clearance has also been measured using a mixture of vegetable charcoal powder and 3% saccharin to demonstrate a delay in patients with CRS as compared to normal, hypertrophied inferior turbinates and septal deviation (1100).
4.3.8.1.2. Ciliary beat frequency
Specific measurements of ciliary activity using a phase contrast microscope with photometric cell (1101, 1102) have been used in a number of studies to evaluate therapeutic success (1103, 1104) (Evidence Level IIb). The normal range from the inferior turbinate is over 8Hz but these techniques are available in only a few centres to which those suspected of primary ciliary dyskinesia are referred. The final gold standard of ciliary function involves culture techniques for 6 weeks (1105).
4.3.8.1.3. Electron microscopy
This may be used to confirm the presence of specific inherited disorders of the cilia as in primary ciliary dyskinesia (1106).
4.3.8.1.4. Nitric oxide
This metabolite found in the upper and lower respiratory tract is a sensitive indicator of the presence of inflammation and ciliary dysfunction, being high with inflammation and low in ciliary dyskinesia It requires little patient co-operation and is quick and easy to perform using chemiluminescence, but the availability of measuring equipment at present limits its use. The majority of nitric oxide is made in the sinuses (chest < 20 ppb, nose 400-900 ppb, sinuses 20 25 ppm) using an LR 2000 Logan Sinclair nitric oxide gas analyser (values may differ with different machines). Less than 100ppb from the upper and <10ppb from the lower respiratory tract would be highly suspicious of PCD. However, whilst very low levels in the nose can indicate primary ciliary dyskinesia, they may also be due to significant sinus obstruction eg severe nasal polyposis (849). Conversely elevated levels suggest nasal inflammation but ostiomeatal patency (849) [Evidence Level IIb]. It can be used, as an outcome measure after therapy (16, 1107) (Evidence Level IIa) but variable baseline levels limit its value in the diagnosis and management of ARS and CRS other than to exclude inherited defects in mucociliary clearance.
A wide range of other diagnostic tests are available to assist with the differential diagnosis and to define predisposing and aetiological factors but many are only available in research departments
4.3.8.1. Mucociliary function
4.3.8.1.1. Nasomucociliary clearance
The use of saccharin, dye or radioactive particles to measure mucociliary transit time has been available for nearly thirty years (1097-1099). It allows one to recognize early alterations of sinosinusal homeostasis. Although a crude measure, it has the advantage of considering the entire mucociliary system and is useful if normal (< 35 minutes). However, if it is prolonged, it does not distinguish between primary or secondary causes of ciliary dysfunction. Nasomucociliary clearance has also been measured using a mixture of vegetable charcoal powder and 3% saccharin to demonstrate a delay in patients with CRS as compared to normal, hypertrophied inferior turbinates and septal deviation (1100).
4.3.8.1.2. Ciliary beat frequency
Specific measurements of ciliary activity using a phase contrast microscope with photometric cell (1101, 1102) have been used in a number of studies to evaluate therapeutic success (1103, 1104) (Evidence Level IIb). The normal range from the inferior turbinate is over 8Hz but these techniques are available in only a few centres to which those suspected of primary ciliary dyskinesia are referred. The final gold standard of ciliary function involves culture techniques for 6 weeks (1105).
4.3.8.1.3. Electron microscopy
This may be used to confirm the presence of specific inherited disorders of the cilia as in primary ciliary dyskinesia (1106).
4.3.8.1.4. Nitric oxide
This metabolite found in the upper and lower respiratory tract is a sensitive indicator of the presence of inflammation and ciliary dysfunction, being high with inflammation and low in ciliary dyskinesia It requires little patient co-operation and is quick and easy to perform using chemiluminescence, but the availability of measuring equipment at present limits its use. The majority of nitric oxide is made in the sinuses (chest < 20 ppb, nose 400-900 ppb, sinuses 20 25 ppm) using an LR 2000 Logan Sinclair nitric oxide gas analyser (values may differ with different machines). Less than 100ppb from the upper and <10ppb from the lower respiratory tract would be highly suspicious of PCD. However, whilst very low levels in the nose can indicate primary ciliary dyskinesia, they may also be due to significant sinus obstruction eg severe nasal polyposis (849). Conversely elevated levels suggest nasal inflammation but ostiomeatal patency (849) [Evidence Level IIb]. It can be used, as an outcome measure after therapy (16, 1107) (Evidence Level IIa) but variable baseline levels limit its value in the diagnosis and management of ARS and CRS other than to exclude inherited defects in mucociliary clearance.
4.3.8.2. Nasal airway assessment
4.3.8.2.1. Nasal inspiratory peak flow
This inexpensive, quick and easy test is a useful estimate of airflow which can be performed at home as well as in the hospital setting. However, it measures both sides together and has little direct role in the assessment of chronic rhinosinusitis. It could be used to assess gross reduction in nasal polyposis and compares well with rhinomanometry (1108, 1109) (Evidence Level IIb). However, peak nasal inspiratory flow (PNIF) does appear to correlate with nasal obstruction symptoms (1110). Normative data is now available in an adult Caucasian population (1111) and for children and adolescents in Brazil and the Netherlands (1112, 1113). Expiratory peak flow is less often used as mucus is expelled into the mask and the technique may be associated with eustachian dysfunction. There is a relationship between NIPF and oral pulmonary expiratory flow (PEF) in that the greater the value of PEF, the greater the NIPF (1114). A minimally clinically important difference of 20L/min has been shown for NIPF (1115) (Evidence Level IIa).
4.3.8.2.2. Rhinomanometry (active anterior and posterior)
The measurement of nasal airway resistance by assessing nasal flow at a constant pressure is again of limited usefulness in chronic rhinosinusitis and nasal polyposis but can be useful in confirming that improvement in nasal congestion is the result of reduction in inflammation in the middle meatus rather than mechanical obstruction (1103) (Evidence Level IIb). The long term mean coefficient of variation (CV) for test-retest over a five month period has been shown to be 27% compared to a shortterm CV of 7-17% within one hour which limits its usefulness (1116) (Evidence Level IIa).
4.3.8.2.3. Acoustic rhinometry
The distortion of a sound wave by nasal topography allows quantification of area at fixed points in the nose from which volume may be derived. Standardisation of the technique has been recommended (1117) and it is a useful test of nasal patency especially in children as little active co-operation is required (1118-1120). It can be used to demonstrate subtle changes, both as a result of medical and surgical intervention, comparable to or better than CT scanning (16, 1109, 1121-1123) (Evidence Level IIa).
4.3.8.2.4. Rhinostereometry
This also measures subtle changes in mucosal swelling, largely in the inferior turbinates (1124, 1125) (Evidence Level IIb) and is therefore not directly applicable to assessment of chronic rhinosinusitis and nasal polyposis.
4.3.8.2.1. Nasal inspiratory peak flow
This inexpensive, quick and easy test is a useful estimate of airflow which can be performed at home as well as in the hospital setting. However, it measures both sides together and has little direct role in the assessment of chronic rhinosinusitis. It could be used to assess gross reduction in nasal polyposis and compares well with rhinomanometry (1108, 1109) (Evidence Level IIb). However, peak nasal inspiratory flow (PNIF) does appear to correlate with nasal obstruction symptoms (1110). Normative data is now available in an adult Caucasian population (1111) and for children and adolescents in Brazil and the Netherlands (1112, 1113). Expiratory peak flow is less often used as mucus is expelled into the mask and the technique may be associated with eustachian dysfunction. There is a relationship between NIPF and oral pulmonary expiratory flow (PEF) in that the greater the value of PEF, the greater the NIPF (1114). A minimally clinically important difference of 20L/min has been shown for NIPF (1115) (Evidence Level IIa).
4.3.8.2.2. Rhinomanometry (active anterior and posterior)
The measurement of nasal airway resistance by assessing nasal flow at a constant pressure is again of limited usefulness in chronic rhinosinusitis and nasal polyposis but can be useful in confirming that improvement in nasal congestion is the result of reduction in inflammation in the middle meatus rather than mechanical obstruction (1103) (Evidence Level IIb). The long term mean coefficient of variation (CV) for test-retest over a five month period has been shown to be 27% compared to a shortterm CV of 7-17% within one hour which limits its usefulness (1116) (Evidence Level IIa).
4.3.8.2.3. Acoustic rhinometry
The distortion of a sound wave by nasal topography allows quantification of area at fixed points in the nose from which volume may be derived. Standardisation of the technique has been recommended (1117) and it is a useful test of nasal patency especially in children as little active co-operation is required (1118-1120). It can be used to demonstrate subtle changes, both as a result of medical and surgical intervention, comparable to or better than CT scanning (16, 1109, 1121-1123) (Evidence Level IIa).
4.3.8.2.4. Rhinostereometry
This also measures subtle changes in mucosal swelling, largely in the inferior turbinates (1124, 1125) (Evidence Level IIb) and is therefore not directly applicable to assessment of chronic rhinosinusitis and nasal polyposis.
4.3.8.3. Olfaction
4.3.8.3.1. Threshold Testing
Fluctuations in the sense of smell are associated with chronic rhinosinusitis. This may due to a conductive loss secondary to obstruction (1110), or to degenerative alterations in the olfactory mucosa due to the disease or its treatment eg. repeated nasal surgery (1). Recently, transgenic technology has also demonstrated that local inflammation within the olfactory epithelium can generate olfactory loss (1126). The estimation of olfactory thresholds by the presentation of serial dilutions of pure odourants such as pm carbinol have been used in a number of studies (1104, 1121, 1127-1129) (Evidence Levels IIb, III).
4.3.8.3.2. Other quantitative olfactory testing
Scratch and sniff test using patches impregnated with microencapsulated odorants are available (256) and have been utilised in studies of both chronic rhinosinusitis and nasal polyposis (1109). A cruder screening test, the Zurich Smell Diskette test may also be used and has the advantage of pictorial representation of the items (1130, 1131). Also on a national footing, the Barcelona Smell Test has been developed, comprising 24 odorants and has been compared with the Zurich Smell Diskette Test (245). More complex tests exist (1132) e.g. 'Sniff 'n' sticks' which combines threshold, discrimination and odour identification and which can be used to perform unilateral testing (1133). A combined supra-threshold detection and identification test has been devised as a crosscultural tool in the European population (1134), the results of which are presented in an appendix in EPOS2007 (1) (Evidence Level III).
Sources of some commercially available and validated olfactory tests are also mentioned in the appendix (1).
4.3.8.4. Aspirin and other challenges
Objective experiments to differentiate patient groups according to severity or aetiology of rhinosinusitis have been done by provocation with histamine or metacholine (1135, 1136) which test mucosal hyper-reactivity. The tests can differentiate sub-populations with statistical significance, but because of considerable overlap of results, the tests have not achieved the equivalent position as the corresponding tests in asthma diagnosis.
Diagnosis of aspirin hypersensitivity is important as it will provide the patient with a long list of common drugs that must not be taken to avoid the risk of a severe reaction. It diagnoses a particular type of asthma and sinonasal disease and allows the choice of a specific therapy ie aspirin desensitisation. The oral aspirin challenge test was introduced to clinical practice in the early 1970s (1137) and since then has been validated (1138- 1140). An inhalation test was introduced in 1977, which is safer and faster to perform than the oral one though less sensitive (1141-1143). Unlike the oral challenge, it does not produce systemic reactions. Nasal challenge was introduced in the 1980s (1144, 1145) and is recommended for patients with predominantly nasal symptoms or those in whom oral or inhaled tests are contraindicated because of the asthma severity. A negative nasal challenge should be followed by oral challenge. Lysine aspirin, the truly soluble form of aspirin must be used for both respiratory routes. Test procedures have been reviewed in detail (1146) and the sensitivity and specificity of the tests are shown in Table 4.3.3. The sensitivity of nasal challenge has been shown to be increased by prolonging the detection time from 2 to 3 hours (1147). The challenges must be performed under medical supervision and results measured with symptoms, acoustic rhinometry or anterior rhinomanometry and pulmonary function.
4.3.8.3.1. Threshold Testing
Fluctuations in the sense of smell are associated with chronic rhinosinusitis. This may due to a conductive loss secondary to obstruction (1110), or to degenerative alterations in the olfactory mucosa due to the disease or its treatment eg. repeated nasal surgery (1). Recently, transgenic technology has also demonstrated that local inflammation within the olfactory epithelium can generate olfactory loss (1126). The estimation of olfactory thresholds by the presentation of serial dilutions of pure odourants such as pm carbinol have been used in a number of studies (1104, 1121, 1127-1129) (Evidence Levels IIb, III).
4.3.8.3.2. Other quantitative olfactory testing
Scratch and sniff test using patches impregnated with microencapsulated odorants are available (256) and have been utilised in studies of both chronic rhinosinusitis and nasal polyposis (1109). A cruder screening test, the Zurich Smell Diskette test may also be used and has the advantage of pictorial representation of the items (1130, 1131). Also on a national footing, the Barcelona Smell Test has been developed, comprising 24 odorants and has been compared with the Zurich Smell Diskette Test (245). More complex tests exist (1132) e.g. 'Sniff 'n' sticks' which combines threshold, discrimination and odour identification and which can be used to perform unilateral testing (1133). A combined supra-threshold detection and identification test has been devised as a crosscultural tool in the European population (1134), the results of which are presented in an appendix in EPOS2007 (1) (Evidence Level III).
Sources of some commercially available and validated olfactory tests are also mentioned in the appendix (1).
4.3.8.4. Aspirin and other challenges
Objective experiments to differentiate patient groups according to severity or aetiology of rhinosinusitis have been done by provocation with histamine or metacholine (1135, 1136) which test mucosal hyper-reactivity. The tests can differentiate sub-populations with statistical significance, but because of considerable overlap of results, the tests have not achieved the equivalent position as the corresponding tests in asthma diagnosis.
Diagnosis of aspirin hypersensitivity is important as it will provide the patient with a long list of common drugs that must not be taken to avoid the risk of a severe reaction. It diagnoses a particular type of asthma and sinonasal disease and allows the choice of a specific therapy ie aspirin desensitisation. The oral aspirin challenge test was introduced to clinical practice in the early 1970s (1137) and since then has been validated (1138- 1140). An inhalation test was introduced in 1977, which is safer and faster to perform than the oral one though less sensitive (1141-1143). Unlike the oral challenge, it does not produce systemic reactions. Nasal challenge was introduced in the 1980s (1144, 1145) and is recommended for patients with predominantly nasal symptoms or those in whom oral or inhaled tests are contraindicated because of the asthma severity. A negative nasal challenge should be followed by oral challenge. Lysine aspirin, the truly soluble form of aspirin must be used for both respiratory routes. Test procedures have been reviewed in detail (1146) and the sensitivity and specificity of the tests are shown in Table 4.3.3. The sensitivity of nasal challenge has been shown to be increased by prolonging the detection time from 2 to 3 hours (1147). The challenges must be performed under medical supervision and results measured with symptoms, acoustic rhinometry or anterior rhinomanometry and pulmonary function.
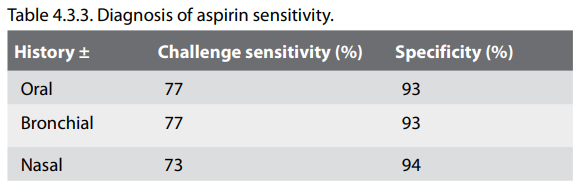
4.3.9. Laboratory assesments – C-reactive protein
(CRP)
Known since 1930, C-reactive protein is part of the acute phase response proteins. Its principal properties are short half-life (6-8 h), rapid response (within 6 hours) and high levels (x500 normal) after injury. It activates the classical complement pathway, leading to bacterial opsonization. Studies have shown that the CRP value is useful in the diagnosis of bacterial infections (1148). However, among patients suspected of an infectious disease, CRP levels up to 100 mg/l are compatible with all types of infections (bacterial, viral, fungal, and protozoal) (1149).
Sequential CRP measurements will have greater diagnostic value than a single measurement and changes of the CRP values often reflect the clinical course. When used in general practice the diagnostic value of CRP is found to be high in adults with pneumonia, sinusitis and tonsillitis. Measurement of CRP is an important diagnostic test but the analysis should not standalone but be evaluated together with the patient's history and clinical examination (1150). .
CRP is most reliably used for exclusion of bacterial infection: two values less than 10 mg/l and 8 12 hours apart can be taken to exclude bacterial infection (1149) and is now available in general practice at the point-of-care (249).
A range of other blood tests may be undertaken in specific cases as part of the differential diagnosis. This may include full blood count including eosinophils, ESR, evaluation of renal, liver and thyroid function, humoral immunity markers (immunoglobulins, IgG subclasses, IgE and IgG to Aspergillis, specific antibody levels to tetanus, haemophilus, pneumococcus) and response to immunization if low, cellular immunity markers (T and B cell and ratios), HIV, ACE and ANCA (1151).
Known since 1930, C-reactive protein is part of the acute phase response proteins. Its principal properties are short half-life (6-8 h), rapid response (within 6 hours) and high levels (x500 normal) after injury. It activates the classical complement pathway, leading to bacterial opsonization. Studies have shown that the CRP value is useful in the diagnosis of bacterial infections (1148). However, among patients suspected of an infectious disease, CRP levels up to 100 mg/l are compatible with all types of infections (bacterial, viral, fungal, and protozoal) (1149).
Sequential CRP measurements will have greater diagnostic value than a single measurement and changes of the CRP values often reflect the clinical course. When used in general practice the diagnostic value of CRP is found to be high in adults with pneumonia, sinusitis and tonsillitis. Measurement of CRP is an important diagnostic test but the analysis should not standalone but be evaluated together with the patient's history and clinical examination (1150). .
CRP is most reliably used for exclusion of bacterial infection: two values less than 10 mg/l and 8 12 hours apart can be taken to exclude bacterial infection (1149) and is now available in general practice at the point-of-care (249).
A range of other blood tests may be undertaken in specific cases as part of the differential diagnosis. This may include full blood count including eosinophils, ESR, evaluation of renal, liver and thyroid function, humoral immunity markers (immunoglobulins, IgG subclasses, IgE and IgG to Aspergillis, specific antibody levels to tetanus, haemophilus, pneumococcus) and response to immunization if low, cellular immunity markers (T and B cell and ratios), HIV, ACE and ANCA (1151).
4.3.10. Validation of subjective symptoms assessment
4.3.10.1. Nasal obstruction
Validation of subjective assessment of nasal obstruction or stuffiness has been done by studying the relationship between subjective and objective evaluation methods for functional nasal obstruction. However, the patient's interpretation of nasal blockage has been shown to vary from true mechanical obstruction of airflow to the sensation of fullness in the midface (1152). Generally the subjective sensation of nasal obstruction and rhinomanometric or nasal peak flow evaluations show a good intra-individual correlation in a number of studies considering normal controls, patients with structural abnormalities, hyperreactivity or infective rhinitis (1153-1158). However, there are also some studies where this correlation is not seen (1159) or the correlation was poor (1160-1162). The inter-patient variation in subjective scoring suggests that every nose is "individually calibrated", which makes inter-patient comparisons less reliable but still significant (1153, 1155).
Subjective nasal obstruction correlates better with objective functional measurements of nasal airflow resistance (rinomanometry, peak flow) than with measurements of nasal cavity width, such as acoustic rhinometry (1158, 1163). Rhinomanometry has been shown to correlate with subjective symptom scoring with and without decongestion (1164). In healthy individuals there is a poor correlation between acoustic rhinometry and subjective nasal obstruction scores, though these are better in congested subjects (1165). Nasal obstruction can also be assessed objectively by tests using personal nasal peak flow instruments, inspiratory or expiratory, which patients can take home or to their work place and do measurements at any desired time intervals. Subjective assessment of nasal obstruction is a well-validated criterion.
4.3.10.2. Nasal discharge
Techniques for objective assessment of nasal discharge are not as good as for nasal obstruction: counting the nose blowings in a diary card or using a new handkerchief from a counted reservoir for each blow and possibly collecting the used handkerchieves in plastic bags for weighing have been used in acute infective rhinitis (1166) and in "autonomic (previously termed vasomotor) rhinitis" (1167).
Validating correlation studies between "objective" discharge measures (collecting and measuring amount or weight of nasal secretion as drops, by suction, or using hygroscopic paper strips etc) and subjective scoring of nasal discharge or postnasal drip has not been done.
4.3.10.3. Smell abnormalities
Fluctuations in the sense of smell are associated with chronic rhinosinusitis. This may be due to mucosal obstruction of the olfactory niche (conductive loss) and/or degenerative alterations in the olfactory mucosa due to the disease or its treatment eg repeated nasal surgery.
Subjective scoring of olfaction is a commonly used assessment method. In validating clinical settings subjective scores have been found to correlate significantly to objective olfactory threshold and qualitative tests in normal population, rhinosinusitis with and without nasal polyps and other disease conditions (243-245, 1168-1172).
4.3.10.4. Facial pain and pressure
Facial or dental pain, especially unilateral, have been found to be predictors of acute maxillary sinusitis with fluid retention in patients with a suspicion of infection, when validated by maxillary antral aspiration (236) or paranasal sinus radiographs (1173). The importance of facial pain as a cardinal sign of chronic rhinosinusitis has also been called into question (See section 4.4) (1174) where the symptoms are more diffuse and fluctuate rendering the clinical correlation of facial pain and pressure scorings against objective assessments unconvincing. In a study correlating symptoms with CT Lund-Mackay scores, patients presenting with facial pain as a primary symptom were more likely with a score of 0 or 1 (ie normal) on CT (1175, 1176). Poor correlation between facial pain localisation and the affected paranasal sinus CT pathology in patients with supposed infection, both acute and chronic, has been reported (1177). However, rhinosinusitis disease specific quality of life studies also include facial pain-related parameters, which have been validated (1178).
4.3.10.1. Nasal obstruction
Validation of subjective assessment of nasal obstruction or stuffiness has been done by studying the relationship between subjective and objective evaluation methods for functional nasal obstruction. However, the patient's interpretation of nasal blockage has been shown to vary from true mechanical obstruction of airflow to the sensation of fullness in the midface (1152). Generally the subjective sensation of nasal obstruction and rhinomanometric or nasal peak flow evaluations show a good intra-individual correlation in a number of studies considering normal controls, patients with structural abnormalities, hyperreactivity or infective rhinitis (1153-1158). However, there are also some studies where this correlation is not seen (1159) or the correlation was poor (1160-1162). The inter-patient variation in subjective scoring suggests that every nose is "individually calibrated", which makes inter-patient comparisons less reliable but still significant (1153, 1155).
Subjective nasal obstruction correlates better with objective functional measurements of nasal airflow resistance (rinomanometry, peak flow) than with measurements of nasal cavity width, such as acoustic rhinometry (1158, 1163). Rhinomanometry has been shown to correlate with subjective symptom scoring with and without decongestion (1164). In healthy individuals there is a poor correlation between acoustic rhinometry and subjective nasal obstruction scores, though these are better in congested subjects (1165). Nasal obstruction can also be assessed objectively by tests using personal nasal peak flow instruments, inspiratory or expiratory, which patients can take home or to their work place and do measurements at any desired time intervals. Subjective assessment of nasal obstruction is a well-validated criterion.
4.3.10.2. Nasal discharge
Techniques for objective assessment of nasal discharge are not as good as for nasal obstruction: counting the nose blowings in a diary card or using a new handkerchief from a counted reservoir for each blow and possibly collecting the used handkerchieves in plastic bags for weighing have been used in acute infective rhinitis (1166) and in "autonomic (previously termed vasomotor) rhinitis" (1167).
Validating correlation studies between "objective" discharge measures (collecting and measuring amount or weight of nasal secretion as drops, by suction, or using hygroscopic paper strips etc) and subjective scoring of nasal discharge or postnasal drip has not been done.
4.3.10.3. Smell abnormalities
Fluctuations in the sense of smell are associated with chronic rhinosinusitis. This may be due to mucosal obstruction of the olfactory niche (conductive loss) and/or degenerative alterations in the olfactory mucosa due to the disease or its treatment eg repeated nasal surgery.
Subjective scoring of olfaction is a commonly used assessment method. In validating clinical settings subjective scores have been found to correlate significantly to objective olfactory threshold and qualitative tests in normal population, rhinosinusitis with and without nasal polyps and other disease conditions (243-245, 1168-1172).
4.3.10.4. Facial pain and pressure
Facial or dental pain, especially unilateral, have been found to be predictors of acute maxillary sinusitis with fluid retention in patients with a suspicion of infection, when validated by maxillary antral aspiration (236) or paranasal sinus radiographs (1173). The importance of facial pain as a cardinal sign of chronic rhinosinusitis has also been called into question (See section 4.4) (1174) where the symptoms are more diffuse and fluctuate rendering the clinical correlation of facial pain and pressure scorings against objective assessments unconvincing. In a study correlating symptoms with CT Lund-Mackay scores, patients presenting with facial pain as a primary symptom were more likely with a score of 0 or 1 (ie normal) on CT (1175, 1176). Poor correlation between facial pain localisation and the affected paranasal sinus CT pathology in patients with supposed infection, both acute and chronic, has been reported (1177). However, rhinosinusitis disease specific quality of life studies also include facial pain-related parameters, which have been validated (1178).
4.3.10. Correlation between patient-reported symptoms and
objective measures
Several publications have demonstrated the lack of correlation between patient rates measures of symptom severity in chronic rhinosinusitis and objective measures, such as the radiological Lund-Mackay scoring system (1179-1182). Similarly a recent systematic review has demonstrated no correlation between sensation of nasal obstruction and measurements of crosssectional airflow using rhinometry (1183).
The relationship between the biological burden of disease and symptoms is complex. Physiological variables can be profoundly abnormal in some asymptomatic patients, while others may report severe symptoms in the absence of change in biological markers of disease – for example a patient may present with severe symptoms of CRS in the face of minimal disease on cross-sectional imaging, while another may be virtually asymptomatic despite pansinusitis on CT. Studies in many other medical specialties demonstrate that patient reported measures of symptoms are poorly correlated with clinical measures. In studies of benign prostatic hypertrophy there was only a modest association between urodynamic indices of obstruction and obstructive symptoms (1184). Studies of asthma and COPD have found little or no correlation between subjective dyspnoea and FEV1 (1185).
It is proposed that patients' symptoms and quality of life are the result of an interaction between many factors, in which biological or physiological variables are only a piece of the final jigsaw (1186). Disease severity is modified by the interactions between many patient factors. For example, studies have shown the gender appears to modify symptom severity in sinonasal disease, with women reporting higher SNOT-20 (1187) scores than men for the same level of disease severity on cross sectional imaging. AERD, depression (1188) and ethnicity (1189) have also be shown to worsen baseline QOL in CRS. Cultural expectations, age, socio-economic status and additional comorbidities are amongst other factors that may modify the impact of disease. Clinicians probably overestimate the impact that measurable biological variables have on symptoms and functioning. It is perhaps not surprising that there should be little correlation between a patient-based symptom severity-scoring systems. The absence of correlation does not suggest that either patient rated or objective scores are invalid, but that they are measuring different aspects of the disease process, and therefore are useful adjuncts in outcome measurement.
For the majority of rhinological complaints where reducing the impact of symptoms on the quality of life of the patient is the primary aim of treatment, patient-rated measures are usually more useful in guiding treatment and measuring the resulting outcome. Clinician-rated measures may however provide more useful feedback to the surgeon in terms of technique. There are also occasions when clinician-rated measures are important to guide whether treatment is likely to be successful; and to confirm if the clinical aim is achieved.
Several publications have demonstrated the lack of correlation between patient rates measures of symptom severity in chronic rhinosinusitis and objective measures, such as the radiological Lund-Mackay scoring system (1179-1182). Similarly a recent systematic review has demonstrated no correlation between sensation of nasal obstruction and measurements of crosssectional airflow using rhinometry (1183).
The relationship between the biological burden of disease and symptoms is complex. Physiological variables can be profoundly abnormal in some asymptomatic patients, while others may report severe symptoms in the absence of change in biological markers of disease – for example a patient may present with severe symptoms of CRS in the face of minimal disease on cross-sectional imaging, while another may be virtually asymptomatic despite pansinusitis on CT. Studies in many other medical specialties demonstrate that patient reported measures of symptoms are poorly correlated with clinical measures. In studies of benign prostatic hypertrophy there was only a modest association between urodynamic indices of obstruction and obstructive symptoms (1184). Studies of asthma and COPD have found little or no correlation between subjective dyspnoea and FEV1 (1185).
It is proposed that patients' symptoms and quality of life are the result of an interaction between many factors, in which biological or physiological variables are only a piece of the final jigsaw (1186). Disease severity is modified by the interactions between many patient factors. For example, studies have shown the gender appears to modify symptom severity in sinonasal disease, with women reporting higher SNOT-20 (1187) scores than men for the same level of disease severity on cross sectional imaging. AERD, depression (1188) and ethnicity (1189) have also be shown to worsen baseline QOL in CRS. Cultural expectations, age, socio-economic status and additional comorbidities are amongst other factors that may modify the impact of disease. Clinicians probably overestimate the impact that measurable biological variables have on symptoms and functioning. It is perhaps not surprising that there should be little correlation between a patient-based symptom severity-scoring systems. The absence of correlation does not suggest that either patient rated or objective scores are invalid, but that they are measuring different aspects of the disease process, and therefore are useful adjuncts in outcome measurement.
For the majority of rhinological complaints where reducing the impact of symptoms on the quality of life of the patient is the primary aim of treatment, patient-rated measures are usually more useful in guiding treatment and measuring the resulting outcome. Clinician-rated measures may however provide more useful feedback to the surgeon in terms of technique. There are also occasions when clinician-rated measures are important to guide whether treatment is likely to be successful; and to confirm if the clinical aim is achieved.
4.4. Facial Pain
4.4.1. Summary
The majority of patients who present with facial pain and headaches believe they have 'sinus trouble'. There is an increasing awareness that neurological causes are responsible for a large proportion of patient's headache and facial pain. The vast majority of patients who present with a symmetrical frontal or temporal headache, sometimes with an occipital component, have tension type headache. Unilateral, episodic headaches are often vascular in origin. Rhinosinusitis rarely causes headache, let alone facial pain, except when there is an acute bacterial infection when the sinus in question cannot drain - and it is usually unilateral and severe. These patients usually have a history of a viral upper respiratory infection immediately before this and they have pyrexia with unilateral nasal obstruction. The vast majority of patients with acute rhinosinusitis respond to antibiotics. More than two episodes of genuine bacterial rhinosinusitis in one year should be investigated for evidence of poor immunity. Patients with chronic bacterial rhinosinusitis rarely have any pain unless the sinus ostia are blocked and their symptoms are similar to acute rhinosinusitis.
With the advent of nasal endoscopy and computerised tomography, along with the finding that many patients' symptoms of headache or facial pain persist after sinus surgery it has become apparent that many patient's symptoms are not due to their sinuses. It is also relevant that over 80% of patients with purulent secretions visible at nasal endoscopy have no headache or facial pain. Even if patients with intermittent symptoms of headache or facial pain, and who believe that it is due to infection, are asked to attend the clinic when they are symptomatic the majority are found not to have any evidence of infection and a neurological cause is responsible. Over 90% of self-diagnosed and doctor-diagnosed sinus headaches meet the International Headache Society criteria for migraines and yet 60% receive an antibiotic prescription. Over 40% of migraine sufferers had at least one unilateral nasal symptom of congestion or rhinorrhoea or ocular lacrimation, redness or swelling during an attack, which can confuse the picture but these episodes do not last longer than 72 hours. In cases of headache or facial pain secondary to genuine rhinosinusitis, there are usually endoscopic signs of disease, and these patients almost invariably have coexisting symptoms of nasal obstruction, hyposmia and/or a purulent nasal discharge. An interdisciplinary consensus group recently agreed that "the majority of sinus headaches can actually be classified as migraines" and that "unnecessary diagnostic studies, surgical interventions, and medical treatments are often the result of the inappropriate diagnosis of sinus headache".
Other unilateral, episodic headaches are also vascular in origin, being hemicrania continua, cluster headache or paroxysmal hemicrania – although the latter two comprise more periorbital pain than headache. A relatively recently described condition, which affects about a third of patients with facial pain seen in ENT clinics, is midfacial segment pain. This is a version of tension-type headache that affects the midface and its features include a symmetrical sensation of pressure or tightness that can involve the areas of the nasion, under the bridge of the nose, either side of the nose, the peri- or retro-orbital regions, or across the cheeks. The symptoms of tension type headache often coexist. There may be hyperaesthesia of the skin and soft tissues over the affected area. There are no consistent exacerbating or relieving factors. There are no nasal symptoms (note that approximately 20% of most populations have intermittent or persistent allergic rhinitis, which may occur incidentally in this condition). The majority of patients with this condition respond to low dose amitriptyline, but usually require up to 6 weeks of 10 mg (occasionally 20 mg) at night before it works. Amitriptyline should then be continued for 6 months before stopping it, and the 20% whose symptoms return when they stop it need to restart it if the pain returns.
Patients with facial pain who have no objective evidence of sinus disease (endoscopy negative) are very unlikely to be helped by nasal medical or surgical treatment. In these patients, other diagnoses should be considered and an appropriate medical treatment tried.
A comprehensive examination including nasendoscopy is highly desirable if medical nasal treatment directed at sinusitis has failed in order to confirm or refute the diagnosis of sinusitis.
4.4.1. Summary
The majority of patients who present with facial pain and headaches believe they have 'sinus trouble'. There is an increasing awareness that neurological causes are responsible for a large proportion of patient's headache and facial pain. The vast majority of patients who present with a symmetrical frontal or temporal headache, sometimes with an occipital component, have tension type headache. Unilateral, episodic headaches are often vascular in origin. Rhinosinusitis rarely causes headache, let alone facial pain, except when there is an acute bacterial infection when the sinus in question cannot drain - and it is usually unilateral and severe. These patients usually have a history of a viral upper respiratory infection immediately before this and they have pyrexia with unilateral nasal obstruction. The vast majority of patients with acute rhinosinusitis respond to antibiotics. More than two episodes of genuine bacterial rhinosinusitis in one year should be investigated for evidence of poor immunity. Patients with chronic bacterial rhinosinusitis rarely have any pain unless the sinus ostia are blocked and their symptoms are similar to acute rhinosinusitis.
With the advent of nasal endoscopy and computerised tomography, along with the finding that many patients' symptoms of headache or facial pain persist after sinus surgery it has become apparent that many patient's symptoms are not due to their sinuses. It is also relevant that over 80% of patients with purulent secretions visible at nasal endoscopy have no headache or facial pain. Even if patients with intermittent symptoms of headache or facial pain, and who believe that it is due to infection, are asked to attend the clinic when they are symptomatic the majority are found not to have any evidence of infection and a neurological cause is responsible. Over 90% of self-diagnosed and doctor-diagnosed sinus headaches meet the International Headache Society criteria for migraines and yet 60% receive an antibiotic prescription. Over 40% of migraine sufferers had at least one unilateral nasal symptom of congestion or rhinorrhoea or ocular lacrimation, redness or swelling during an attack, which can confuse the picture but these episodes do not last longer than 72 hours. In cases of headache or facial pain secondary to genuine rhinosinusitis, there are usually endoscopic signs of disease, and these patients almost invariably have coexisting symptoms of nasal obstruction, hyposmia and/or a purulent nasal discharge. An interdisciplinary consensus group recently agreed that "the majority of sinus headaches can actually be classified as migraines" and that "unnecessary diagnostic studies, surgical interventions, and medical treatments are often the result of the inappropriate diagnosis of sinus headache".
Other unilateral, episodic headaches are also vascular in origin, being hemicrania continua, cluster headache or paroxysmal hemicrania – although the latter two comprise more periorbital pain than headache. A relatively recently described condition, which affects about a third of patients with facial pain seen in ENT clinics, is midfacial segment pain. This is a version of tension-type headache that affects the midface and its features include a symmetrical sensation of pressure or tightness that can involve the areas of the nasion, under the bridge of the nose, either side of the nose, the peri- or retro-orbital regions, or across the cheeks. The symptoms of tension type headache often coexist. There may be hyperaesthesia of the skin and soft tissues over the affected area. There are no consistent exacerbating or relieving factors. There are no nasal symptoms (note that approximately 20% of most populations have intermittent or persistent allergic rhinitis, which may occur incidentally in this condition). The majority of patients with this condition respond to low dose amitriptyline, but usually require up to 6 weeks of 10 mg (occasionally 20 mg) at night before it works. Amitriptyline should then be continued for 6 months before stopping it, and the 20% whose symptoms return when they stop it need to restart it if the pain returns.
Patients with facial pain who have no objective evidence of sinus disease (endoscopy negative) are very unlikely to be helped by nasal medical or surgical treatment. In these patients, other diagnoses should be considered and an appropriate medical treatment tried.
A comprehensive examination including nasendoscopy is highly desirable if medical nasal treatment directed at sinusitis has failed in order to confirm or refute the diagnosis of sinusitis.
4.4.2.Introduction
Otorhinolaryngologists see many patients with facial pain. They have the equipment to help diagnose whose facial pain is due to paranasal sinus disease or, as important, whose is not. This is vital as so many patients and their physicians mistakenly attribute their pain as being due to rhinosinusitis, when this is not the case. In the group of people who are referred to an ORL surgeon with a presumptive diagnosis of rhinosinusitis as the cause for their facial pain, only 1 in 8 patients are found to have pain attributable to their sinus disease (1076). "Significant caution is needed when considering surgery in those patients (with facial pain) because of high long-term failure rates and the eventual identification of other causes of the pain in many cases" (1190). This does not mean that rhinosinusitis does not cause facial pain, rather that caution is needed before making the link to this diagnosis.
Facial pain without any other nasal symptoms is unlikely to be due to rhinosinusitis.
4.4.3. Sinogenic facial pain
Before describing the characteristics of facial pain secondary to sinusitis it is worthy of note that over 80% of patients with purulent secretions visible at nasal endoscopy have no facial pain and those that do have it during an acute exacerbation (1191) and the majority of patients with nasal polyposis do not have pain (1192). Children with chronic rhinosinusitis very rarely complain of facial pain, even in the presence of florid purulent secretions. Also of note is the fact that a significant proportion of patients in several series have persisting facial pain after endoscopic sinus surgery (1174, 1193). In other words not only does chronic rhinosinusitis not usually cause facial pain but facial pain is not synonymous with rhinosinusitis. Interestingly the IHS (International Headache Society) classification says "chronic rhinosinusitis is not validated as a cause of headache or facial pain unless relapsing into an acute stage" (1194).
4.4.3.1. Bacterial rhinosinusitis
Acute bacterial rhinosinusitis usually follows an acute viral upper respiratory tract infection and if there is pain it is usually unilateral, severe, associated with pyrexia in about 50% and they have nasal obstruction. In maxillary sinusitis unilateral facial and dental pain are good predictors of true infection and this has been validated in studies using maxillary sinus aspiration (236) (Evidence Level III). This differs from chronic rhinosinusitis where there is a poor correlation between the site of facial pain and evidence of sinus pathology (1177, 1195) (Evidence Level III). An increase in the severity of pain on bending forward has traditionally been thought to be diagnostic of sinusitis but this is non-specific and it can occur in many other types of facial pain.
Coexisting nasal obstruction and/or clear rhinorrhoea can occur along with various types of vascular facial pain but these are normally short lived and rarely last longer than 48 hours.
The key points in the history of sinus related pain are an exacerbation of pain during an upper respiratory tract infection, an association with rhinological symptoms, worse when flying or skiing and a response to antibiotic medical treatment. It is important to realise that many types of facial pain are vascular in origin and last less than 72 hours so that a patient with this type of pain might presume that their pain has responded to an antibiotic when it would have resolved within this time frame in any event. A good history is vital in arriving at a correct diagnosis. There is no diagnostic investigation that can make a diagnosis in most neurological causes of facial pain other than analysing the patients' symptom complex in the light of their examination and response to treatment. "When patients present with a headache and that they believe to be related to allergies and sinus problems, the clinical interview is often orientated by them in a way that supports their assumption" (1196).
Normal nasal endoscopy makes it very unlikely that a person's facial pain is due to rhinosinusitis
4.4.3.2. Examination
In acute frontal sinusitis the patient is often pyrexial and has tenderness on the medial side of the orbital floor under the supraorbital ridge where the frontal sinus is thinnest. Endoscopic examination shows marked hyperaemia of the nasal mucosa and purulent secretions are often visible. Acute sphenoiditis is uncommon and said to cause pain at the vertex of the head but pain can be referred to the temporal region or whole head. Facial swelling other than that caused by periorbital cellulitis, cavernous sinus thrombosis or subgaleal infection usually results from dental sepsis (440, 455, 1197) (Evidence level III). A normal nasal cavity, showing no evidence of middle meatal mucopus or inflammatory changes makes a diagnosis of sinogenic pain most unlikely, particularly if the patient is currently in pain or has had pain within the past few days. If the patient or surgeon are in doubt because the patient is asymptomatic on the day they are seen and their nasal endoscopy is normal in the clinic, it is often useful to review them and repeat the nasendoscopy when they have pain in order to clarify the diagnosis.
If a patient complains of constant symmetrical facial pain then midfacial segment pain should be excluded.
It is extremely rare for patients to have endoscopic evidence of inflammation or infection when they return with their pain. Even the presence of inflammatory changes or infection does not indicate with any certainty that the pain is sinogenic as it can occasionally be incidental (1076) (Evidence level III). If it is incidental this will become apparent as the patients pain will persist after their sinusitis has resolved.
Nasal endoscopy has better specificity than CT in diagnosing whether someone has rhinosinusitis.
Otorhinolaryngologists see many patients with facial pain. They have the equipment to help diagnose whose facial pain is due to paranasal sinus disease or, as important, whose is not. This is vital as so many patients and their physicians mistakenly attribute their pain as being due to rhinosinusitis, when this is not the case. In the group of people who are referred to an ORL surgeon with a presumptive diagnosis of rhinosinusitis as the cause for their facial pain, only 1 in 8 patients are found to have pain attributable to their sinus disease (1076). "Significant caution is needed when considering surgery in those patients (with facial pain) because of high long-term failure rates and the eventual identification of other causes of the pain in many cases" (1190). This does not mean that rhinosinusitis does not cause facial pain, rather that caution is needed before making the link to this diagnosis.
Facial pain without any other nasal symptoms is unlikely to be due to rhinosinusitis.
4.4.3. Sinogenic facial pain
Before describing the characteristics of facial pain secondary to sinusitis it is worthy of note that over 80% of patients with purulent secretions visible at nasal endoscopy have no facial pain and those that do have it during an acute exacerbation (1191) and the majority of patients with nasal polyposis do not have pain (1192). Children with chronic rhinosinusitis very rarely complain of facial pain, even in the presence of florid purulent secretions. Also of note is the fact that a significant proportion of patients in several series have persisting facial pain after endoscopic sinus surgery (1174, 1193). In other words not only does chronic rhinosinusitis not usually cause facial pain but facial pain is not synonymous with rhinosinusitis. Interestingly the IHS (International Headache Society) classification says "chronic rhinosinusitis is not validated as a cause of headache or facial pain unless relapsing into an acute stage" (1194).
4.4.3.1. Bacterial rhinosinusitis
Acute bacterial rhinosinusitis usually follows an acute viral upper respiratory tract infection and if there is pain it is usually unilateral, severe, associated with pyrexia in about 50% and they have nasal obstruction. In maxillary sinusitis unilateral facial and dental pain are good predictors of true infection and this has been validated in studies using maxillary sinus aspiration (236) (Evidence Level III). This differs from chronic rhinosinusitis where there is a poor correlation between the site of facial pain and evidence of sinus pathology (1177, 1195) (Evidence Level III). An increase in the severity of pain on bending forward has traditionally been thought to be diagnostic of sinusitis but this is non-specific and it can occur in many other types of facial pain.
Coexisting nasal obstruction and/or clear rhinorrhoea can occur along with various types of vascular facial pain but these are normally short lived and rarely last longer than 48 hours.
The key points in the history of sinus related pain are an exacerbation of pain during an upper respiratory tract infection, an association with rhinological symptoms, worse when flying or skiing and a response to antibiotic medical treatment. It is important to realise that many types of facial pain are vascular in origin and last less than 72 hours so that a patient with this type of pain might presume that their pain has responded to an antibiotic when it would have resolved within this time frame in any event. A good history is vital in arriving at a correct diagnosis. There is no diagnostic investigation that can make a diagnosis in most neurological causes of facial pain other than analysing the patients' symptom complex in the light of their examination and response to treatment. "When patients present with a headache and that they believe to be related to allergies and sinus problems, the clinical interview is often orientated by them in a way that supports their assumption" (1196).
Normal nasal endoscopy makes it very unlikely that a person's facial pain is due to rhinosinusitis
4.4.3.2. Examination
In acute frontal sinusitis the patient is often pyrexial and has tenderness on the medial side of the orbital floor under the supraorbital ridge where the frontal sinus is thinnest. Endoscopic examination shows marked hyperaemia of the nasal mucosa and purulent secretions are often visible. Acute sphenoiditis is uncommon and said to cause pain at the vertex of the head but pain can be referred to the temporal region or whole head. Facial swelling other than that caused by periorbital cellulitis, cavernous sinus thrombosis or subgaleal infection usually results from dental sepsis (440, 455, 1197) (Evidence level III). A normal nasal cavity, showing no evidence of middle meatal mucopus or inflammatory changes makes a diagnosis of sinogenic pain most unlikely, particularly if the patient is currently in pain or has had pain within the past few days. If the patient or surgeon are in doubt because the patient is asymptomatic on the day they are seen and their nasal endoscopy is normal in the clinic, it is often useful to review them and repeat the nasendoscopy when they have pain in order to clarify the diagnosis.
If a patient complains of constant symmetrical facial pain then midfacial segment pain should be excluded.
It is extremely rare for patients to have endoscopic evidence of inflammation or infection when they return with their pain. Even the presence of inflammatory changes or infection does not indicate with any certainty that the pain is sinogenic as it can occasionally be incidental (1076) (Evidence level III). If it is incidental this will become apparent as the patients pain will persist after their sinusitis has resolved.
Nasal endoscopy has better specificity than CT in diagnosing whether someone has rhinosinusitis.
4.4.3.3. Investigations
Plain sinus x-rays are very insensitive and non-specific in diagnosing chronic sinusitis The interpretation of changes on the sinuses with computerised tomography (CT) scans must also be treated with caution. Approximately 30% of asymptomatic patients will demonstrate mucosal thickening in one or more sinuses on CT scanning. The presence of this finding is certainly not an indication that pain is sinogenic in origin (277, 570, 1177, 1195, 1198, 1199) (Evidence level III) However, a clear CT makes it very unlikely there is any rhinosinusitis. In one study of 305 patients who met the American Academy Taskforce clinical criteria for chronic rhinosinusitis, of whom 154 had facial pain, they found that 60% had normal sinuses thereby questioning the diagnosis and selection criteria. More recent guidelines include endoscopic findings +/- CT changes to confirm the diagnosis (5, 1175) (Evidence level III). Care should be taken in making the diagnosis of recurrent acute sinusitis as this is very unusual and patients who have two or more bacterial sinus infections within 12 months should be investigated for an immune deficiency (560, 1200, 1201).
If a patient complains of recurrent acute rhinosinusitis, yet they are clear when you see them, ask them to return when they are symptomatic. Recurrent bacterial rhinosinusitis is rare. Most of these patients with recurrent facial pain have a vascular aetiology for their pain
4.4.3.4. Medical treatment
The majority of patients with bacterial sinusitis respond to treatment with antibiotics. The common pathogens are streptococcus pneumoniae and haemophilus influenzae and less commonly S. aureus and M. catarrhalis (296, 1202-1204) (Evidence level III), various streptococci, and a minority have anaerobes such as bacteroides and anaerobic streptococci. In chronic bacterial rhinosinusitis, defined by its persistence over 12 weeks (1205), anaerobes (1206) and staphylococci (639) (Evidence level III) are more prevalent.
4.4.3.5. Surgery
Surgery is normally effective in helping many symptoms in patients with genuine rhinosinusitis unresponsive to medical treatment but specific care is needed when the symptom of facial pain is concerned.An analysis of 10 series of endoscopic sinus surgery of 1,713 patients showed a mean improvement rate of 91%, taking a range of symptoms into account (1207) but this series does not specifically address the issue of facial pain. Many studies have looked at quality of life, or encompass a range of nasal symptoms and pathologies and do not provide a sufficient breakdown of the different symptoms to analyze the effect of surgery on facial pain (1208-1217). Studies that have looked at symptoms of facial pain and pressure in sinusitis show that between 56-77% of patients who have facial pain are better after sinus surgery (1217, 1218) (Evidence level IIb). However, these studies they do not claim very good results in the complete resolution of facial pain. One study with a validated outcome score showed an improvement in facial pain and headache after endoscopic sinus surgery in patients with facial pain caused by sinusitis (1219) (Evidence level III). It is important to ensure that the diagnosis of rhinosinusitis is correct before embarking on surgery.
Plain sinus x-rays are very insensitive and non-specific in diagnosing chronic sinusitis The interpretation of changes on the sinuses with computerised tomography (CT) scans must also be treated with caution. Approximately 30% of asymptomatic patients will demonstrate mucosal thickening in one or more sinuses on CT scanning. The presence of this finding is certainly not an indication that pain is sinogenic in origin (277, 570, 1177, 1195, 1198, 1199) (Evidence level III) However, a clear CT makes it very unlikely there is any rhinosinusitis. In one study of 305 patients who met the American Academy Taskforce clinical criteria for chronic rhinosinusitis, of whom 154 had facial pain, they found that 60% had normal sinuses thereby questioning the diagnosis and selection criteria. More recent guidelines include endoscopic findings +/- CT changes to confirm the diagnosis (5, 1175) (Evidence level III). Care should be taken in making the diagnosis of recurrent acute sinusitis as this is very unusual and patients who have two or more bacterial sinus infections within 12 months should be investigated for an immune deficiency (560, 1200, 1201).
If a patient complains of recurrent acute rhinosinusitis, yet they are clear when you see them, ask them to return when they are symptomatic. Recurrent bacterial rhinosinusitis is rare. Most of these patients with recurrent facial pain have a vascular aetiology for their pain
4.4.3.4. Medical treatment
The majority of patients with bacterial sinusitis respond to treatment with antibiotics. The common pathogens are streptococcus pneumoniae and haemophilus influenzae and less commonly S. aureus and M. catarrhalis (296, 1202-1204) (Evidence level III), various streptococci, and a minority have anaerobes such as bacteroides and anaerobic streptococci. In chronic bacterial rhinosinusitis, defined by its persistence over 12 weeks (1205), anaerobes (1206) and staphylococci (639) (Evidence level III) are more prevalent.
4.4.3.5. Surgery
Surgery is normally effective in helping many symptoms in patients with genuine rhinosinusitis unresponsive to medical treatment but specific care is needed when the symptom of facial pain is concerned.An analysis of 10 series of endoscopic sinus surgery of 1,713 patients showed a mean improvement rate of 91%, taking a range of symptoms into account (1207) but this series does not specifically address the issue of facial pain. Many studies have looked at quality of life, or encompass a range of nasal symptoms and pathologies and do not provide a sufficient breakdown of the different symptoms to analyze the effect of surgery on facial pain (1208-1217). Studies that have looked at symptoms of facial pain and pressure in sinusitis show that between 56-77% of patients who have facial pain are better after sinus surgery (1217, 1218) (Evidence level IIb). However, these studies they do not claim very good results in the complete resolution of facial pain. One study with a validated outcome score showed an improvement in facial pain and headache after endoscopic sinus surgery in patients with facial pain caused by sinusitis (1219) (Evidence level III). It is important to ensure that the diagnosis of rhinosinusitis is correct before embarking on surgery.
4.4.3.6. Facial pain and CRS with nasal polyps
(CRSwNP).
Chronic rhinosinusitis with or without clear evidence of a bacterial infection is often painless, except during an acute exacerbation precipitated by an upper respiratory tract infection or induced by barotrauma. CRSwNP patients rarely have facial pain, even with opaque sinuses on CT, unless there is an acute exacerbation with obstruction of the sinus ostia (1192) (Evidence level III).
4.4.3.7. Other diseases of the nose or paranasal sinuses that cause facial pain
Although tumours rarely present with facial pain, constant, progressive pain, particularly if associated with other suspicious symptoms or neurological signs should alert the clinician. A thorough examination and appropriate imaging is mandatory to exclude the possibility of a tumour. Stretching of the arterial tree which, supplies the proximal portions of the cranial nerves and the dura within 1 cm of any venous sinus induces a headache but can cause facial pain. The supratentorial vessels and dura refer pain to the ophthalmic division of the trigeminal nerve. Infratentorial structures refer pain to the distribution of the glossopharyngeal nerve and vagus, along with the upper three cervical nerve dermatomes. Space-occupying lesions such as meningiomas, angiomas and intracerebral metastases can induce facial pain by irritation of the trigeminal nerve along its intracerebral course. Syringobulbia, syphilis and multiple sclerosis are rarer causes of central lesions, which may cause facial pain. Raised intracranial pressure produces a bursting headache, which is worse on coughing or straining and is associated with effortless vomiting. The fundi can show papilloedema in around a third of patients. Lesions in the posterior cranial fossa produce occipital and upper neck pain, while supratentorial lesions with raised intracranial pressure produce pain at the vertex or over the frontal and temporal region. Cerebrovascular accidents can cause such pain, but these symptoms may only present when the other more distressing signs and symptoms of a stroke are resolving. They are particularly severe when part of the thalamus has been infracted
Carcinoma of the maxilla is uncommon. Patients unfortunately often present late when the disease has spread beyond the confines of the sinuses. Unilateral serosanguinous nasal discharge and obstruction is the most frequent presentation. Less common symptoms are infraorbital paraesthesiae, loose teeth or ill-fitting dentures, proptosis, deformity of the cheek, epiphora, nasal obstruction or epistaxis. Pain is usually a late feature. Occasionally adenoid cystic carcinoma can present with pain in the distribution of the trigeminal ganglion or its branches.
Chronic rhinosinusitis with or without clear evidence of a bacterial infection is often painless, except during an acute exacerbation precipitated by an upper respiratory tract infection or induced by barotrauma. CRSwNP patients rarely have facial pain, even with opaque sinuses on CT, unless there is an acute exacerbation with obstruction of the sinus ostia (1192) (Evidence level III).
4.4.3.7. Other diseases of the nose or paranasal sinuses that cause facial pain
Although tumours rarely present with facial pain, constant, progressive pain, particularly if associated with other suspicious symptoms or neurological signs should alert the clinician. A thorough examination and appropriate imaging is mandatory to exclude the possibility of a tumour. Stretching of the arterial tree which, supplies the proximal portions of the cranial nerves and the dura within 1 cm of any venous sinus induces a headache but can cause facial pain. The supratentorial vessels and dura refer pain to the ophthalmic division of the trigeminal nerve. Infratentorial structures refer pain to the distribution of the glossopharyngeal nerve and vagus, along with the upper three cervical nerve dermatomes. Space-occupying lesions such as meningiomas, angiomas and intracerebral metastases can induce facial pain by irritation of the trigeminal nerve along its intracerebral course. Syringobulbia, syphilis and multiple sclerosis are rarer causes of central lesions, which may cause facial pain. Raised intracranial pressure produces a bursting headache, which is worse on coughing or straining and is associated with effortless vomiting. The fundi can show papilloedema in around a third of patients. Lesions in the posterior cranial fossa produce occipital and upper neck pain, while supratentorial lesions with raised intracranial pressure produce pain at the vertex or over the frontal and temporal region. Cerebrovascular accidents can cause such pain, but these symptoms may only present when the other more distressing signs and symptoms of a stroke are resolving. They are particularly severe when part of the thalamus has been infracted
Carcinoma of the maxilla is uncommon. Patients unfortunately often present late when the disease has spread beyond the confines of the sinuses. Unilateral serosanguinous nasal discharge and obstruction is the most frequent presentation. Less common symptoms are infraorbital paraesthesiae, loose teeth or ill-fitting dentures, proptosis, deformity of the cheek, epiphora, nasal obstruction or epistaxis. Pain is usually a late feature. Occasionally adenoid cystic carcinoma can present with pain in the distribution of the trigeminal ganglion or its branches.
4.4.4. Non-sinogenic facial pain
4.4.4.1. General comments on the main categories of non-sinogenic facial pain
Only about 1 in 8 patients attending a rhinology clinic have pain that is attributable to rhinosinusitis (1076, 1289,1204). (Evidence level III). The remainder of patients who have non-sinogenic pain have migraine or its variations, tension type headache/ midfacial segment pain, trigeminal-autonomic cephalgias, neuropathic pain or other specific neurological conditions. Clinical examination and diagnostic tests rarely help to make a diagnosis (rare exceptions include an MRI in multiple sclerosis or brainstem tumours, and PET scans can shown abnormalities in the hypothalamus in cluster headache). In facial pain a diagnosis is primarily made on the basis of the history and response to treatment. The following broad characteristics are used to categorise the main types of facial pain:
4.4.4.2. Migraine, Trigeminal-autonomic cephalgias, Cluster headache, Paroxysmal Hemicrania, SUNCT syndrome (short lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing)
Vascular pain of various types can be associated with autonomic rhinological symptoms such as nasal congestion and rhinorrhoea and this has lead to confusion in arriving at a correct diagnosis as many patients understandably believe that these symptoms are synonymous with rhinosinusitis. The prevalence of trigeminal autonomic symptoms is approximately 27% (1220) (Evidence level III). Other causes of facial pain include atypical forms of migraine (1221-1224) cluster headache, paroxysmal hemicrania and atypical facial pain.
Migraine has been defined by the IHS (1194) as an episodic headache lasting 4-72 hours with certain distinguishing features. The diagnostic criteria for migraine are shown in Table 4.4.4. These include throbbing head pain in attacks, often with a prodromal state and usually preceeded by an aura, which frequently contains visual phenomena. Migraine is occasionally isolated to the face and a minority can have pain confined to the periorbital area, and rarely affect the cheek and nose alone. (1225). The pain is typically unilateral but may be bilateral. Nausea, vomiting, photophobia and phonophobia often accompany the pain. The prevalence of migraine that involves the face is approximately 9% of the whole migraine population and occasionally it can be isolated to the face (1226, 1227). Patients who have migraine that involves the face have more trigeminoautonomic symptoms than other migraine patients (1226). The condition has 2 main forms. One type, migraine without aura (previously called common migraine) affects almost 75% of migraine sufferers. It is characterised by a headache, which can be severe and is typically unilateral, sharp, pulsating and often accompanied by nausea, photophobia or phonophobia. Symptoms last 4 to72 hours. There is no premonition. The second type, migraine with aura (previously called classic migraine) affects 25% of migraine sufferers. The attacks are preceded by neurological symptoms such as visual disturbances or numbness. It is three times more common in women and there is often a family history. Stress release, diet, the premenstrual state and barometric pressure can induce attacks. This system of classification is conservative, and it is recognised that many patients fall outside these criteria yet have migraine (1228). Thus, the diagnostic criteria are highly specific but less sensitive. Other conditions have some migrainous features such as cluster headache and paroxysmal hemicrania. These however, have cohesive groups of symptoms that allow them to be categorised separately. However, many patients with facial pain do not neatly fit any diagnostic criteria. Some have migrainous features such as nausea, an aura, or facial flushing. The proposed theories of the cause of migraine have swung between being a primarily vascular or neural mechanism. Griggs and Nutt suggested migraine may be part of the spectrum of diseases known as channelopathies - disorders involving voltage-gated channels (1228). The genetic component of migraine may be explained by the identification of migraine genes in familial hemiplegic migraine that affect the Ca2+ channei (1229). In recent years, neuroimaging of the primary headache syndromes, such as migraine and cluster headaches, has begun to provide a glimpse of the neuroanatomical and physiological basis of these conditions. Functional imaging with positron emission tomography and magnetic resonance angiography (MRA) have documented activation in the midbrain, pons (1230) and hypothalamic grey matter in cluster headache (1231, 1232). Work by Goadsby and colleagues suggest activation of the trigeminal innervation of the cranial circulation due to vasoactive peptides such as calcium gene related peptide (1233- 1235).
4.4.4.1. General comments on the main categories of non-sinogenic facial pain
Only about 1 in 8 patients attending a rhinology clinic have pain that is attributable to rhinosinusitis (1076, 1289,1204). (Evidence level III). The remainder of patients who have non-sinogenic pain have migraine or its variations, tension type headache/ midfacial segment pain, trigeminal-autonomic cephalgias, neuropathic pain or other specific neurological conditions. Clinical examination and diagnostic tests rarely help to make a diagnosis (rare exceptions include an MRI in multiple sclerosis or brainstem tumours, and PET scans can shown abnormalities in the hypothalamus in cluster headache). In facial pain a diagnosis is primarily made on the basis of the history and response to treatment. The following broad characteristics are used to categorise the main types of facial pain:
4.4.4.2. Migraine, Trigeminal-autonomic cephalgias, Cluster headache, Paroxysmal Hemicrania, SUNCT syndrome (short lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing)
Vascular pain of various types can be associated with autonomic rhinological symptoms such as nasal congestion and rhinorrhoea and this has lead to confusion in arriving at a correct diagnosis as many patients understandably believe that these symptoms are synonymous with rhinosinusitis. The prevalence of trigeminal autonomic symptoms is approximately 27% (1220) (Evidence level III). Other causes of facial pain include atypical forms of migraine (1221-1224) cluster headache, paroxysmal hemicrania and atypical facial pain.
Migraine has been defined by the IHS (1194) as an episodic headache lasting 4-72 hours with certain distinguishing features. The diagnostic criteria for migraine are shown in Table 4.4.4. These include throbbing head pain in attacks, often with a prodromal state and usually preceeded by an aura, which frequently contains visual phenomena. Migraine is occasionally isolated to the face and a minority can have pain confined to the periorbital area, and rarely affect the cheek and nose alone. (1225). The pain is typically unilateral but may be bilateral. Nausea, vomiting, photophobia and phonophobia often accompany the pain. The prevalence of migraine that involves the face is approximately 9% of the whole migraine population and occasionally it can be isolated to the face (1226, 1227). Patients who have migraine that involves the face have more trigeminoautonomic symptoms than other migraine patients (1226). The condition has 2 main forms. One type, migraine without aura (previously called common migraine) affects almost 75% of migraine sufferers. It is characterised by a headache, which can be severe and is typically unilateral, sharp, pulsating and often accompanied by nausea, photophobia or phonophobia. Symptoms last 4 to72 hours. There is no premonition. The second type, migraine with aura (previously called classic migraine) affects 25% of migraine sufferers. The attacks are preceded by neurological symptoms such as visual disturbances or numbness. It is three times more common in women and there is often a family history. Stress release, diet, the premenstrual state and barometric pressure can induce attacks. This system of classification is conservative, and it is recognised that many patients fall outside these criteria yet have migraine (1228). Thus, the diagnostic criteria are highly specific but less sensitive. Other conditions have some migrainous features such as cluster headache and paroxysmal hemicrania. These however, have cohesive groups of symptoms that allow them to be categorised separately. However, many patients with facial pain do not neatly fit any diagnostic criteria. Some have migrainous features such as nausea, an aura, or facial flushing. The proposed theories of the cause of migraine have swung between being a primarily vascular or neural mechanism. Griggs and Nutt suggested migraine may be part of the spectrum of diseases known as channelopathies - disorders involving voltage-gated channels (1228). The genetic component of migraine may be explained by the identification of migraine genes in familial hemiplegic migraine that affect the Ca2+ channei (1229). In recent years, neuroimaging of the primary headache syndromes, such as migraine and cluster headaches, has begun to provide a glimpse of the neuroanatomical and physiological basis of these conditions. Functional imaging with positron emission tomography and magnetic resonance angiography (MRA) have documented activation in the midbrain, pons (1230) and hypothalamic grey matter in cluster headache (1231, 1232). Work by Goadsby and colleagues suggest activation of the trigeminal innervation of the cranial circulation due to vasoactive peptides such as calcium gene related peptide (1233- 1235).
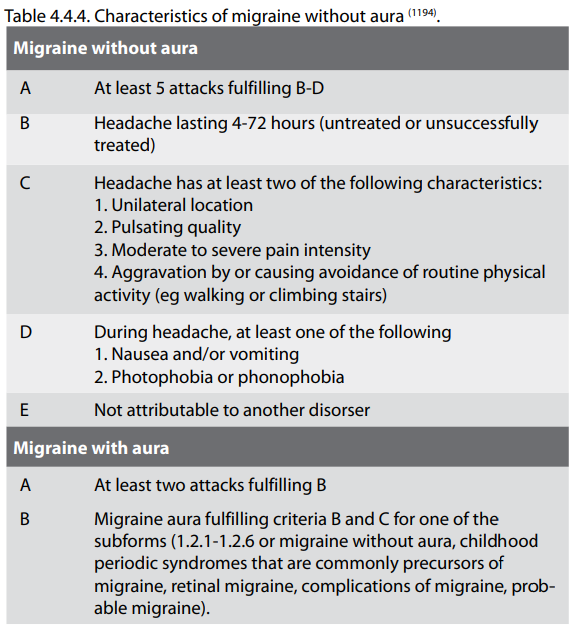
A primary dysfunction of the mid-brain endogenous
antinociceptive system (periaqueductal grey and dorsal raphe
nucleus and the neural control of cerebral blood flow) seems to
be responsible (1236). Neuroimaging has reconciled previous
theories that migraine was solely vascular and they now suggest
that it is a neurovascular headache and an epiphenomenon of
trigeminal activation.
It appears that whilst vascular input predominates in migraine and myofascial nociception prevails in tension type pain, but there is a great deal of overlap. The pioneering work by Olesen and colleagues suggests a neurovascular mechanism (1237) and this is supported by the finding that approximately 50% of patients with tension type headache also have migraine. They proposed a vascular-supraspinal-myogenic model that integrates the effects of pericranial myofascial afferents, activation of peripheral nociceptors from cephalic arteries, and convergence on the caudal nucleus of the trigeminal, along with qualitative changes in the central nervous system. A recent study showed an increase in functional MRI blood oxygen dependant levels in the thalamus during migraine attacks with allodynia, the experience of ordinarily nonpainful stimuli as painful, or hyperaesthesia (1238).
The management of migraine begins with providing information to the patient, the identification and avoidance of aggravating factors. Regular sleep, exercise and a diet that avoids aggravating factors will help many patients although there is no objective data to support this assertion. An assessment must be made on the severity based on a frequency diary, intensity of pain and degree of disability. Pharmacological treatment consists of the management of acute attacks and preventative treatment. Current literature suggests preventative treatment should be considered if symptoms occur more than three times a month with a duration of symptoms more than 48 hours, and if there is a prolonged aura or failure to reduce acute symptoms (1239-1241) (Evidence level III). The options available for medication are shown in Table 4.4.3.
It appears that whilst vascular input predominates in migraine and myofascial nociception prevails in tension type pain, but there is a great deal of overlap. The pioneering work by Olesen and colleagues suggests a neurovascular mechanism (1237) and this is supported by the finding that approximately 50% of patients with tension type headache also have migraine. They proposed a vascular-supraspinal-myogenic model that integrates the effects of pericranial myofascial afferents, activation of peripheral nociceptors from cephalic arteries, and convergence on the caudal nucleus of the trigeminal, along with qualitative changes in the central nervous system. A recent study showed an increase in functional MRI blood oxygen dependant levels in the thalamus during migraine attacks with allodynia, the experience of ordinarily nonpainful stimuli as painful, or hyperaesthesia (1238).
The management of migraine begins with providing information to the patient, the identification and avoidance of aggravating factors. Regular sleep, exercise and a diet that avoids aggravating factors will help many patients although there is no objective data to support this assertion. An assessment must be made on the severity based on a frequency diary, intensity of pain and degree of disability. Pharmacological treatment consists of the management of acute attacks and preventative treatment. Current literature suggests preventative treatment should be considered if symptoms occur more than three times a month with a duration of symptoms more than 48 hours, and if there is a prolonged aura or failure to reduce acute symptoms (1239-1241) (Evidence level III). The options available for medication are shown in Table 4.4.3.
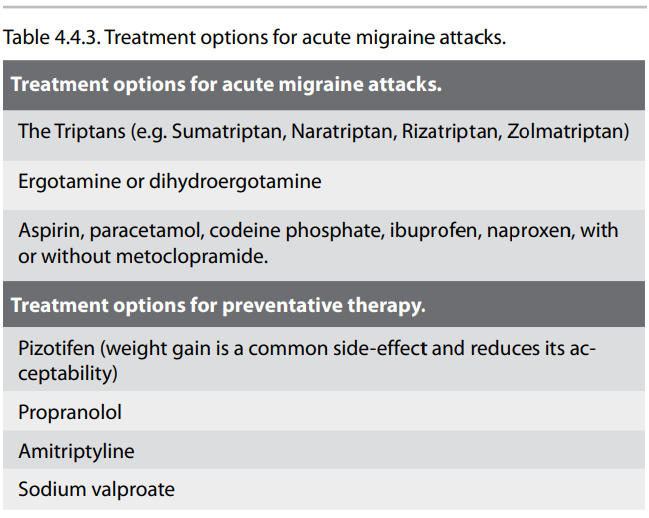
Acute anti-migraine therapy is most likely to be beneficial if
started early in an attack and prophylactic anti-migrainous
medication may need to be continued for up to 6 weeks before its
beneficial effects occur. Treatment can be divided into
nonspecific and specific. The former consists of analgesia, such
as paracetamol, codeine and aspirin, or non-steroidal
antiinflammatory drugs. An anti-emetic may be added if there is
associated nausea or vomiting. If headaches are severe, specific
anti-migrainous medications can be used. These include the
triptans. Ergotamine has to be carefully prescribed as its
overuse can cause severe headaches. The 5-HT1B/D receptor
agonists, or triptans, are shown to be effective after the
headache begins as long as they are given early (1242) (Evidence
level Ib). These constrict blood vessels, block neurogenic
inflammation and neuropeptide release by a neuronal mechanism of
action. Triptans should be prescribed with caution to patients
with ischaemic heart disease, a history of myocardial
infarction, uncontrolled hypertension or cerebrovascular
disease. Pizotifen is a 5-hydroxytriptamine antagonist that is
very effective in the prophylaxis of migraine but its side
effects include weight gain and drowsiness (1243) (Evidence
level Ib). Propranolol, a betareceptor antagonist, also has some
subclass of 5-HT2 effect (1243) (Evidence level Ib). Patients
with asthma should not be given beta-blockers. Topiramate is
preferred to sodium valproate as a second line drug in the
treatment of migraine (1244) (Evidence level Ib).
4.4.4.3. Cluster Headache
Cluster headaches are defined as a primary neurovascular headache that is both severe and uncommon. It is characterised by recurrent, strictly unilateral attacks of headache that typically wake the patient and are retro-orbital or centred at the medial aspect of the orbit, of great intensity and last up to one hour (not in my experience but feel free to change it). The pain is also accompanied by ipsilateral signs of autonomic dysfunction such as the ipsilateral parasympathetic signs of rhinorrhoea, lacrimation, impaired sweating and sympathetic signs of miosis and ptosis (1245). The most salient feature is its periodicity, which could be circadian or in terms of active or inactive bouts lasting 6-10 weeks annually, separated by clinical remission when the patient is completely pain free for at least 2 weeks between attacks. About 15% to 20% of patients suffer from chronic CH and have no significant remissions. Treatment includes sumatriptan injections, oxygen, and prophylactic treatment includes verapamil, gabapentin, and Pizotifen (1246) (Evidence level IIb).
4.4.4.4. Paroxysmal Hemicrania
Paroxysmal Hemicrania has been described as an excruciating unilateral pain, which is usually ocular, and frontotemporal with short-lasting (2-45 minutes), frequent attacks (usually more than 5 a day). By definition, at least one of the following autonomic symptoms should be present; nasal congestion (42%), rhinorrhoea (36%), lacrimation (62%), conjuctival injection (36%), or rarely ptosis, eyelid oedema, heart rate changes (bradycardia, tachycardia and extrasystoles), increased local sweating, salivation and facial flushing (1247, 1248). Attacks may occur bilaterally even though they are usually more pronounced in the symptomatic side. These last between 5 to 45 minutes on each occasion, and they recur many times, between 7-22 times daily. Remission varied between 3 months to 3 years. Rarely do these headaches develop into the chronic form. The ratio is said to be 4:1 for CPH to episodic PH (1249). Overall, the average age of the onset of PH is usually 30-40 years, but the spectrum range from 6 years old to 81 years old. The episodic form tends to have an earlier age onset (1250).
The condition's complete or rapid response to indomethacin is said to differentiate paroxysmal hemicrania from cluster headache (1251) (Evidence level III). However, recently the inclusion of this 'absolute' response to make it a criterion has been questioned (1252-1254) (Evidence level IV). The majority of patients with PH respond to indomethacin within 24 hours. If not, a trial, which entails increasing the dose to 75 mg daily after 3 days, followed by 150 mg daily after another 3 days has been recommended (1255). Another study by Antonaci et al., recommended the 'Indotest', with an intramuscular injection of 50 mg indomethacin, and the response is monitored to differentiate paroxysmal hemicrania, hemicrania continua (HC) and other headache disorders with which they can be confused (1256). They also noted that this test is a useful tool in the clinical assessment of unilateral headaches by establishing the interval between indomethacin administration and the clinical response.
A need for a persistently high dose may imply a sinister underlying pathology (1257). In cases where indomethacin fails to work, other drugs that have been sugested, include calcium-channel blockers (1253, 1258) naproxen, carbamezapine (1259), and sumatriptan (1260) (Evidence level IV). The main features of PH and CH are listed in Table 4.4.1.
Cluster headaches are defined as a primary neurovascular headache that is both severe and uncommon. It is characterised by recurrent, strictly unilateral attacks of headache that typically wake the patient and are retro-orbital or centred at the medial aspect of the orbit, of great intensity and last up to one hour (not in my experience but feel free to change it). The pain is also accompanied by ipsilateral signs of autonomic dysfunction such as the ipsilateral parasympathetic signs of rhinorrhoea, lacrimation, impaired sweating and sympathetic signs of miosis and ptosis (1245). The most salient feature is its periodicity, which could be circadian or in terms of active or inactive bouts lasting 6-10 weeks annually, separated by clinical remission when the patient is completely pain free for at least 2 weeks between attacks. About 15% to 20% of patients suffer from chronic CH and have no significant remissions. Treatment includes sumatriptan injections, oxygen, and prophylactic treatment includes verapamil, gabapentin, and Pizotifen (1246) (Evidence level IIb).
4.4.4.4. Paroxysmal Hemicrania
Paroxysmal Hemicrania has been described as an excruciating unilateral pain, which is usually ocular, and frontotemporal with short-lasting (2-45 minutes), frequent attacks (usually more than 5 a day). By definition, at least one of the following autonomic symptoms should be present; nasal congestion (42%), rhinorrhoea (36%), lacrimation (62%), conjuctival injection (36%), or rarely ptosis, eyelid oedema, heart rate changes (bradycardia, tachycardia and extrasystoles), increased local sweating, salivation and facial flushing (1247, 1248). Attacks may occur bilaterally even though they are usually more pronounced in the symptomatic side. These last between 5 to 45 minutes on each occasion, and they recur many times, between 7-22 times daily. Remission varied between 3 months to 3 years. Rarely do these headaches develop into the chronic form. The ratio is said to be 4:1 for CPH to episodic PH (1249). Overall, the average age of the onset of PH is usually 30-40 years, but the spectrum range from 6 years old to 81 years old. The episodic form tends to have an earlier age onset (1250).
The condition's complete or rapid response to indomethacin is said to differentiate paroxysmal hemicrania from cluster headache (1251) (Evidence level III). However, recently the inclusion of this 'absolute' response to make it a criterion has been questioned (1252-1254) (Evidence level IV). The majority of patients with PH respond to indomethacin within 24 hours. If not, a trial, which entails increasing the dose to 75 mg daily after 3 days, followed by 150 mg daily after another 3 days has been recommended (1255). Another study by Antonaci et al., recommended the 'Indotest', with an intramuscular injection of 50 mg indomethacin, and the response is monitored to differentiate paroxysmal hemicrania, hemicrania continua (HC) and other headache disorders with which they can be confused (1256). They also noted that this test is a useful tool in the clinical assessment of unilateral headaches by establishing the interval between indomethacin administration and the clinical response.
A need for a persistently high dose may imply a sinister underlying pathology (1257). In cases where indomethacin fails to work, other drugs that have been sugested, include calcium-channel blockers (1253, 1258) naproxen, carbamezapine (1259), and sumatriptan (1260) (Evidence level IV). The main features of PH and CH are listed in Table 4.4.1.
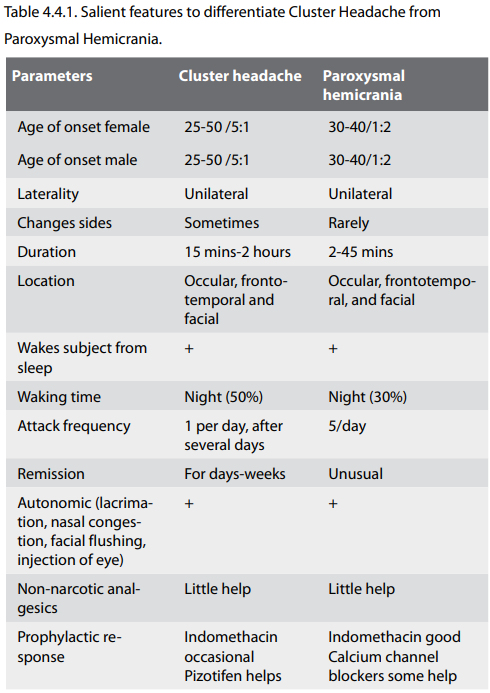
4.4.4.5. Hemicrania Continua
Chronic Paroxysmal Hemicrania and Hemicrania Continua (HC) are two strictly unilateral headache disorders characterised by an absolute response to indomethacin. HC, first described by Sjaastad and Spiering, is a unilateral headache which is moderately severe without side shift, continuous but with fluctuations, with complete resolution of pain with indomethacin, and exacerbations that may be associated with autonomic features such as conjuctival injection, lacrimation, and photophobia to the affected side (1261, 1262) (Evidence level IV).
SUNCT (Short-lasting neuralgiform pain with conjunctival injection and tearing) SUNCT is one of the rarest idiopathic headache syndromes. This is a form of primary headache, marked by trigeminal pain, particularly orbital or periorbital area, associated with autonomic symptoms, in which conjunctival injection and tearing is the most prominent feature. Attacks last between 15 to 60 seconds and recur between 5-30 times an hour. These attacks may be precipitated by chewing movements and ingesting certain foods such as citrus fruits. Treatment is difficult with lamotrigine, carbamezapine or topiramate (1263) (Evidence level IV)
4.4.4.6. Indomethacin-responsive headaches. (Episodic and Chronic Paroxysmal Hemicrania, Remitting and Unremitting Hemicrania Continua, and Benign Cough Headache, Benign Exertional Headache, and sharp short-lived headache pain syndrome)
Indomethacin-responsive headaches are defined as those responding to doses of 25 mg twice daily to 75 mg three times daily, usually having an effect in less than 72 hours from the start of the effective dose. These rare syndromes include Episodic and Chronic Paroxysmal Hemicrania, Remitting and Unremitting Hemicrania Continua, and Benign Cough Headache, Benign Exertional Headache, and sharp short-lived headache pain syndrome. Most of these headaches are provoked by physical stimulation, for example exertion, cough, flexion or extension of the neck (1263).
How indomethacin works for these headaches is unclear. Currently, it is thought that indomethacin reduces the cerebral blood flow (1264), thereby decreasing the load on the presumed phlebotic cavernous sinus which chould be the origin of the pain in CPH attacks (1236) and is results in a decline in cerebral permeability (1265) and cerebrospinal pressure (1266). The antiinflammatory effect of indomethacin on these vessels may also have a role in aborting pain in CPH (1259) (Evidence level IV).
4.4.4.7. Persistent idiopathic facial pain
This is defined as persistent, unilateral facial pain not associated with sensory or physical signs. One study showed that with voxel-based morphometry there was a decrease in grey matter volume in the left anterior cingulated gyrus and left temporoinsular region as well as bilateral motor and sensory areas projecting to the areas that represent the face (1267) (Evidence level IV).
4.4.4.8. Chronic oro-facial pain
A small proportion of patients go on to have chronic pain and a prospective study supports the hypothesis that psychological factors such as anxiety, depression, illness behaviour, somatic symptoms are markers for chronic pain (1268). A multi-disciplinary facial pain clinic supported by a clinical psychologist is very helpful in treating these patients as it not only stops them from "shopping around" but it helps to check that no treatment strategy has been overlooked and after that coping mechanisms can be put in place. Comprehensive pain programmes have been shown to be both therapeutically efficacious and cost-effective in an evidence-based review of the subject (1269). Functional restoration, often through cognitive behaviour therapy, is central to the rehabilitation of most patients with chronic pain, almost whatever the cause. Psychological therapies are similarly helpful in children and adolescents with chronic pain (1270) (Evidence level Ib).
4.4.4.9. "Sinus Headaches"
Headaches that are due to rhinosinusitis are very uncommon and confined to a minority of patients who have acute frontal sinusitis or sphenoiditis. The vast majority of people who present with a symmetrical frontal or temporal headache, sometimes with an occipital component, have tension type headache. Unilateral, episodic headaches are often vascular in origin. The idea that rhinosinusitis can trigger migraine is misplaced as the whole symptom complex is vascular and coexisting nasal congestion is due to vasodilation of the nasal mucosa that is sometimes part of the vascular event. The use of nasal endoscopy and imaging of the paranasal sinuses have advanced our appreciation that these patients are suffering from a vascular event.
Over 90% of self-diagnosed and doctor-diagnosed sinus headaches meet the International Headache Society criteria for migraines and yet 61% receive an antibiotic prescription (1271). One study of 100 patients who believed that they suffered from sinus headache found that 52% had migraine, 11% had chronic migraine associated with medication overuse, 23% had probable migraine, 1% cluster headache, 1% hemicrania continua, 3% secondary to rhinosinusitis, 9% were nonclassifiable (1272). Seventy-six percent of migraine subjects reported pain in the distribution of the second division of the trigeminal nerve (either unilateral or bilateral), 62% experienced bilateral forehead and maxillary pain with their headaches and the most common associated feature was nasal congestion in 56% and rhinorrhoea in 25% (1272). Another study of 1000 patients with headache has as the diagnostic causes migraine, tension-type headache, trigeminal autonomic cephalalgias, cranial neuralgias, trauma, drugs but that sinusitis is very, very rarely the cause (1273). In another study 46% of migraine sufferers attending a tertiary referral centre had at least one unilateral nasal symptom of congestion or rhinorrhoea or ocular lacrimation, redness or swelling during an attack due to the trigeminal-autonomic reflex (1222). Another study found that in self-reported sinus headaches 82% of patients had a significant response to empiric treatment with triptans (1223) (Evidence level IIb). Cady and Schreiber comment that "The concept of sinus disease as a common cause of headache is deeply engrained in the American public, but there is little evidence to support the sinuses as a common cause of disabling headache." (1274). They reported that nearly 90% of participants with self-diagnosed or physician-diagnosed sinus headache met the IHS criteria for migraine-type headache and responded to triptans. They note that during a migrainous episode there is engorgement and erythema of the nasal mucosa along with rhinorrhoea and after subcutaneous sumatriptan both the symptoms and endoscopic signs resolve. Others have found that migraine often affects the face and can be misinterpreted as being due to rhinosinusitis, particularly as symptoms can last 72 hours and that vascular changes in the lining of the nose can also produce nasal obstruction through vasodilatation of the vascular turbinate tissue (1223, 1275). An interdisciplinary consensus group recently agreed that "the majority of sinus headaches can actually be classified as migraines" and that "unnecessary diagnostic studies, surgical interventions, and medical treatments are often the result of the inappropriate diagnosis of sinus headache" (1276). (Table 4.4.2.)
Other conditions that are often considered to induce headache are not sufficiently validated as causes of headache. These include deviation of nasal septum, hypertrophy of turbinates, atrophy of sinus membranes and mucosal contact
Chronic Paroxysmal Hemicrania and Hemicrania Continua (HC) are two strictly unilateral headache disorders characterised by an absolute response to indomethacin. HC, first described by Sjaastad and Spiering, is a unilateral headache which is moderately severe without side shift, continuous but with fluctuations, with complete resolution of pain with indomethacin, and exacerbations that may be associated with autonomic features such as conjuctival injection, lacrimation, and photophobia to the affected side (1261, 1262) (Evidence level IV).
SUNCT (Short-lasting neuralgiform pain with conjunctival injection and tearing) SUNCT is one of the rarest idiopathic headache syndromes. This is a form of primary headache, marked by trigeminal pain, particularly orbital or periorbital area, associated with autonomic symptoms, in which conjunctival injection and tearing is the most prominent feature. Attacks last between 15 to 60 seconds and recur between 5-30 times an hour. These attacks may be precipitated by chewing movements and ingesting certain foods such as citrus fruits. Treatment is difficult with lamotrigine, carbamezapine or topiramate (1263) (Evidence level IV)
4.4.4.6. Indomethacin-responsive headaches. (Episodic and Chronic Paroxysmal Hemicrania, Remitting and Unremitting Hemicrania Continua, and Benign Cough Headache, Benign Exertional Headache, and sharp short-lived headache pain syndrome)
Indomethacin-responsive headaches are defined as those responding to doses of 25 mg twice daily to 75 mg three times daily, usually having an effect in less than 72 hours from the start of the effective dose. These rare syndromes include Episodic and Chronic Paroxysmal Hemicrania, Remitting and Unremitting Hemicrania Continua, and Benign Cough Headache, Benign Exertional Headache, and sharp short-lived headache pain syndrome. Most of these headaches are provoked by physical stimulation, for example exertion, cough, flexion or extension of the neck (1263).
How indomethacin works for these headaches is unclear. Currently, it is thought that indomethacin reduces the cerebral blood flow (1264), thereby decreasing the load on the presumed phlebotic cavernous sinus which chould be the origin of the pain in CPH attacks (1236) and is results in a decline in cerebral permeability (1265) and cerebrospinal pressure (1266). The antiinflammatory effect of indomethacin on these vessels may also have a role in aborting pain in CPH (1259) (Evidence level IV).
4.4.4.7. Persistent idiopathic facial pain
This is defined as persistent, unilateral facial pain not associated with sensory or physical signs. One study showed that with voxel-based morphometry there was a decrease in grey matter volume in the left anterior cingulated gyrus and left temporoinsular region as well as bilateral motor and sensory areas projecting to the areas that represent the face (1267) (Evidence level IV).
4.4.4.8. Chronic oro-facial pain
A small proportion of patients go on to have chronic pain and a prospective study supports the hypothesis that psychological factors such as anxiety, depression, illness behaviour, somatic symptoms are markers for chronic pain (1268). A multi-disciplinary facial pain clinic supported by a clinical psychologist is very helpful in treating these patients as it not only stops them from "shopping around" but it helps to check that no treatment strategy has been overlooked and after that coping mechanisms can be put in place. Comprehensive pain programmes have been shown to be both therapeutically efficacious and cost-effective in an evidence-based review of the subject (1269). Functional restoration, often through cognitive behaviour therapy, is central to the rehabilitation of most patients with chronic pain, almost whatever the cause. Psychological therapies are similarly helpful in children and adolescents with chronic pain (1270) (Evidence level Ib).
4.4.4.9. "Sinus Headaches"
Headaches that are due to rhinosinusitis are very uncommon and confined to a minority of patients who have acute frontal sinusitis or sphenoiditis. The vast majority of people who present with a symmetrical frontal or temporal headache, sometimes with an occipital component, have tension type headache. Unilateral, episodic headaches are often vascular in origin. The idea that rhinosinusitis can trigger migraine is misplaced as the whole symptom complex is vascular and coexisting nasal congestion is due to vasodilation of the nasal mucosa that is sometimes part of the vascular event. The use of nasal endoscopy and imaging of the paranasal sinuses have advanced our appreciation that these patients are suffering from a vascular event.
Over 90% of self-diagnosed and doctor-diagnosed sinus headaches meet the International Headache Society criteria for migraines and yet 61% receive an antibiotic prescription (1271). One study of 100 patients who believed that they suffered from sinus headache found that 52% had migraine, 11% had chronic migraine associated with medication overuse, 23% had probable migraine, 1% cluster headache, 1% hemicrania continua, 3% secondary to rhinosinusitis, 9% were nonclassifiable (1272). Seventy-six percent of migraine subjects reported pain in the distribution of the second division of the trigeminal nerve (either unilateral or bilateral), 62% experienced bilateral forehead and maxillary pain with their headaches and the most common associated feature was nasal congestion in 56% and rhinorrhoea in 25% (1272). Another study of 1000 patients with headache has as the diagnostic causes migraine, tension-type headache, trigeminal autonomic cephalalgias, cranial neuralgias, trauma, drugs but that sinusitis is very, very rarely the cause (1273). In another study 46% of migraine sufferers attending a tertiary referral centre had at least one unilateral nasal symptom of congestion or rhinorrhoea or ocular lacrimation, redness or swelling during an attack due to the trigeminal-autonomic reflex (1222). Another study found that in self-reported sinus headaches 82% of patients had a significant response to empiric treatment with triptans (1223) (Evidence level IIb). Cady and Schreiber comment that "The concept of sinus disease as a common cause of headache is deeply engrained in the American public, but there is little evidence to support the sinuses as a common cause of disabling headache." (1274). They reported that nearly 90% of participants with self-diagnosed or physician-diagnosed sinus headache met the IHS criteria for migraine-type headache and responded to triptans. They note that during a migrainous episode there is engorgement and erythema of the nasal mucosa along with rhinorrhoea and after subcutaneous sumatriptan both the symptoms and endoscopic signs resolve. Others have found that migraine often affects the face and can be misinterpreted as being due to rhinosinusitis, particularly as symptoms can last 72 hours and that vascular changes in the lining of the nose can also produce nasal obstruction through vasodilatation of the vascular turbinate tissue (1223, 1275). An interdisciplinary consensus group recently agreed that "the majority of sinus headaches can actually be classified as migraines" and that "unnecessary diagnostic studies, surgical interventions, and medical treatments are often the result of the inappropriate diagnosis of sinus headache" (1276). (Table 4.4.2.)
Other conditions that are often considered to induce headache are not sufficiently validated as causes of headache. These include deviation of nasal septum, hypertrophy of turbinates, atrophy of sinus membranes and mucosal contact
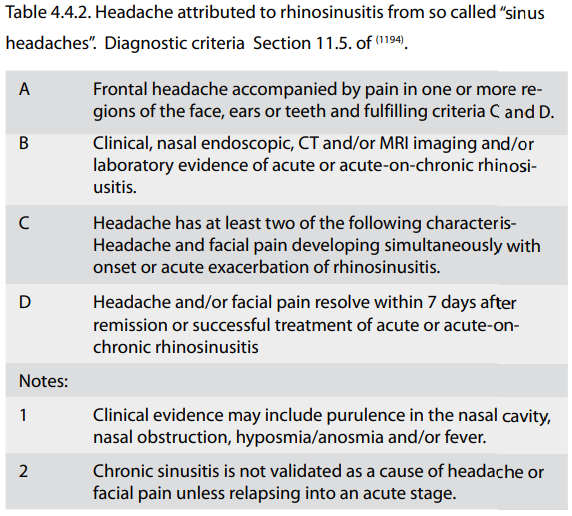
4.4.4.10 Tension type headache
Seventy to eighty percent of the population has headaches every year and 50% have at least one a month, 15% once a week and 5% daily (1277, 1278). The main quality of the pain is one of symmetrical pressure that may be confined to a small area just above the nasion or extend across the whole forehead. There is often an occipital component. There are often no exacerbating or relieving factors although bending forwards can sometimes aggravate them, a symptom often incorrectly said to mean the patient must have rhinosinusitis. There is often some hyperaesthesia of the soft tissues in the area. Patients are often taking many analgesics although they say they do little to help. Analgesic dependant headache can complicate the picture. Withdrawal from analgesics for several weeks alone may be sufficient in this group but is rarely tolerated without starting other treatment for their headache but this is an option. The prevalence of headache increases sharply during the second decade then levels off until the age of 40-50, after which it reduces. The ideas from the Copenhagen group on tensiontype headache (1279, 1280) postulate that central sensitisation of the trigeminal nucleus from either prolonged nociceptive input from a peripheral injury, surgery, inflammation, myofascial nociceptive input, along with psychological or neurological factors that can reduce supraspinal inhibition can contribute to tension-type headache. This concept offers a broader perspective and is a more inclusive method of interpreting. Amitriptyline should be given for six weeks before judging its effect, and should be continued for six months if it has helped (1281, 1282) (Evidence level Ib). The starting dose is 10 mg, and after six weeks if pain is not controlled this can be increased to 20mg (and rarely 50mg are needed). Patients need to be warned of the sedative effects of even at this low dose, but they can be reassured that tolerance usually develops after the first few days. It is our practice to inform patients that amitriptyline is also used in higher doses for other conditions such depression, but that it is not being given for this reason and its effect is unrelated to its analgesic properties, that would take effect much more quickly and normally require 75mgs. It is often reassuring for patients to know that the dose used for depression is some 7 or more times the dose used in tension-type headache and that other antidepressants do not help this condition.
4.4.4.11. Midfacial segment pain
Over the last decade, studies on facial pain have shown that there is a distinct group of patients who have a form of facial neuralgia that has all the characteristics of tension type headache with the exception that it affects the midface (1283). The criteria that comprise midfacial segment pain is:
Seventy to eighty percent of the population has headaches every year and 50% have at least one a month, 15% once a week and 5% daily (1277, 1278). The main quality of the pain is one of symmetrical pressure that may be confined to a small area just above the nasion or extend across the whole forehead. There is often an occipital component. There are often no exacerbating or relieving factors although bending forwards can sometimes aggravate them, a symptom often incorrectly said to mean the patient must have rhinosinusitis. There is often some hyperaesthesia of the soft tissues in the area. Patients are often taking many analgesics although they say they do little to help. Analgesic dependant headache can complicate the picture. Withdrawal from analgesics for several weeks alone may be sufficient in this group but is rarely tolerated without starting other treatment for their headache but this is an option. The prevalence of headache increases sharply during the second decade then levels off until the age of 40-50, after which it reduces. The ideas from the Copenhagen group on tensiontype headache (1279, 1280) postulate that central sensitisation of the trigeminal nucleus from either prolonged nociceptive input from a peripheral injury, surgery, inflammation, myofascial nociceptive input, along with psychological or neurological factors that can reduce supraspinal inhibition can contribute to tension-type headache. This concept offers a broader perspective and is a more inclusive method of interpreting. Amitriptyline should be given for six weeks before judging its effect, and should be continued for six months if it has helped (1281, 1282) (Evidence level Ib). The starting dose is 10 mg, and after six weeks if pain is not controlled this can be increased to 20mg (and rarely 50mg are needed). Patients need to be warned of the sedative effects of even at this low dose, but they can be reassured that tolerance usually develops after the first few days. It is our practice to inform patients that amitriptyline is also used in higher doses for other conditions such depression, but that it is not being given for this reason and its effect is unrelated to its analgesic properties, that would take effect much more quickly and normally require 75mgs. It is often reassuring for patients to know that the dose used for depression is some 7 or more times the dose used in tension-type headache and that other antidepressants do not help this condition.
4.4.4.11. Midfacial segment pain
Over the last decade, studies on facial pain have shown that there is a distinct group of patients who have a form of facial neuralgia that has all the characteristics of tension type headache with the exception that it affects the midface (1283). The criteria that comprise midfacial segment pain is:
- A symmetrical sensation of pressure or tightness. Some patients may say that their nose feels blocked even though they have no nasal airway obstruction.
-
Involves the areas of the nasion, under the bridge of the
nose, either side of the nose, the peri- or retro-orbital
regions, or across the cheeks. The symptoms of tension type
headache often coexist
-
There may be hyperaesthesia of the skin and soft tissues
over the affected area. - Nasal endoscopy is normal.
-
Computerised tomography of the paranasal sinuses is
normal (note a third of asymptomatic patients have
incidental mucosal changes on CT).
-
There are no consistent exacerbating or relieving
factors
-
There are no nasal symptoms (note that
approximately 20% of most populations have
intermittent or persistent allergic rhinitis, which
may occur incidentally in this condition).
4.4.4.12. Analgesic dependant headache
This entity is all too often unrecognised and mismanaged. As has already been mentioned, patients with tension type headache or midfacial segment pain often take a great number of analgesics in spite of the fact that they have little effect. Similarly migraine sufferers can get into a cycle of using an excessive amount of analgesics. Drug-induced headache is usually described as dull, diffuse, and band-like, and usually starts in the early morning. The original headache (migraine or tension headache) has often been present for many years and the regular intake of drugs often started several years before people present. Patients take on average 30 or more tablets per week often containing several different substances. The drugs most often used are caffeine, ergotalkaloids, paracetamol, and pyrazolone derivates. Withdrawal is problematic as patients' symptoms take several weeks to resolve. However, chronic headache disappears or decreases by more than 50% in two-thirds of the patients. Positive predictors for successful treatment are migraine as primary headache, chronic headache lasting less than 10 years, and the regular intake of ergotamine. It is well worth considering if this might be the patient's problem before adding to it with more tablets!
4.4.5. How surgery can influence pain
It is interesting to note that a proportion of patients who mistakenly undergo surgery for non-sinogenic pain experience temporary relief from their symptoms, although their pain returns within a few weeks and nearly always within 9 months. It is hypothesised that the reason for a temporary or partial reduction in their pain is either the effect of cognitive dissonance or the effect of surgical trauma on the afferent fibres going to the trigeminal nucleus, which alters its threshold for spontaneous activity in the short term. In about a third of patients surgery does not significantly affect the pain and in a third the pain is made far worse (1284). Patients whose pain is made worse by surgery may develop a more unpleasant quality to the pain such as burning.The criteria for diagnosing chronic rhinosinusitis vary, but most studies quote more than three nasal symptoms for more than 3 months (490). It is important to note that the inclusion of facial pain/pressure "on its own does not constitute a suggestive history for rhinosinusitis in the absence of another major nasal symptom or sign" (1205). The evidence that a vacuum within a blocked sinus can causes protracted pain is poor. Transient facial pain in patients with other symptoms and signs of rhinosinusitis can occur with acute pressure changes when flying, diving or skiing but this resolves as the pressure within the sinuses equalises within hours through perfusion with the surrounding vasculature. Patients who repeatedly suffer these intermittent symptoms whilst there is a pressure change are often helped by surgery to open the ostia. Persistent blockage of the sinus ostia rarely causes continuous pain for example silent sinus syndrome that is due to a blocked sinus with resorbtion of its contents to the extent that the orbital floor prolapses into the maxillary sinus causes no pain (1285). Endoscopic sinus surgery (ESS) has been advocated by a few workers for facial pain in the absence of endoscopic or CT evidence of sinus disease or anatomical variations Cook et al. advocated ESS on patients with facial pain, which also occurred 'independently' of episodes of rhinosinusitis, with no CT evidence of sinus pathology (1286). Twelve of the 18 patients who underwent surgery in their series had a significant reduction in their pain severity, yet it is significant that, "complete elimination of symptoms was not accomplished in any patient". They had no evidence of ostiomeatal obstruction. If the cause of their pain was due to ostial obstruction then it might be anticipated that surgery would cure their symptoms of pain. This was not the case as they all had residual pain. Similarly Parsons et al. retrospectively described 34 patients with headaches who had contact points removed and found that whilst there was a 91% decrease in intensity and 84% decrease in frequency, 65% had persisting symptoms (1287). One possible reason for a temporary or partial reduction in their pain is either the effect of cognitive dissonance or the effect of surgical trauma on the afferent fibres going to the trigeminal nucleus and this might alter the nucleus and its threshold for spontaneous activity for up to several months as has been found when patients with midfacial segment pain undergo surgery (1174).
4.4.5.1. Post surgical pain/Neuropathic pain
Neuropathic pain arises as a direct consequence of a lesion or disease affecting the somatosensory system (1288). Neuropathic pain is often spontaneous or it can be an abnormal response to a non-painful stimulus. The pain is often deep, burning, gnawing, occasionally stabbing or like an electric shock. It may start after a relatively minor injury or surgical procedure. There may be an altered sensation in the area affected. Acquadro et al. noted that in those patients with preoperative pain who underwent endoscopic sinus surgery, 7% developed new pain, and 2% reported a worsening of their facial pain but none developed de novo pain if they had had no preoperative pain (1289). Indeed to date, there has been few reported cases of facial pain following ESS in previously painfree patients although it may be under-reported (1174, 1284). This fact is surprising given that open sinus surgery, in particular the Caldwell-Luc procedure has long been known to cause de novo facial pain. One study noted this complication in 46% of all patients who had undergone a Caldwell Luc procedure, including some who had no prior facial pain though this may be due to direct trauma to the infraorbital nerve (1290). Trauma causes pain that is mediated by myelinated A delta and unmyelinated C fibres. Prolonged stimulation of these can activate N-methyl-D-aspartate (NMDA) and cause central sensitisation. An alteration in central processing can then lead to an alteration in pain thresholds producing hyperalgesia or even lead to spontaneous firing of neurones and may produce reverberating circuits. It is also possible that antidromic flow in C fibres can cause the release of substance P or that efferent sympathetic flow can release noradrenaline; both these mechanisms have the potential to sensitise peripheral receptors (1291). Trauma can be an initiating factor by either altering the fibres within the trigeminal nucleus or by altering its somatosensory input, thereby altering nocioceptive fibres to or within the caudal nucleus of the trigeminal nerve. These mechanisms, by altering the neuroplasticity of the nerves to and within the trigeminal nucleus, result in neuropathic pain.
Amitriptyline has been shown to be effective in relieving post-traumatic neuralgia (1292) in doses of 75 mg or alternatively gabapentin or pregabalin (1284) (Evidence level III). Duloxetine may help, particularly if there is coexisting anxiety. These need to be given for 6 to 8 weeks before judging if they have helped. A local anaesthetic nerve block can sometimes be successful in blocking localised pain and having a more prolonged benefit. Lignocaine patches over the area can help as can transcutaneous electrical nerve stimulation. The management of patients with pain unresponsive to medical treatment should involve pain coping strategies that involve a pain management team and psychologist and cognitive behaviour therapy. Physical activity, treated depression and anxiety, as well as establishing work or other activities can play an important role. Opiates can help but care is needed in prescribing these as they can lead to tolerance and dependency, which is a further obstacle to recovery.
This entity is all too often unrecognised and mismanaged. As has already been mentioned, patients with tension type headache or midfacial segment pain often take a great number of analgesics in spite of the fact that they have little effect. Similarly migraine sufferers can get into a cycle of using an excessive amount of analgesics. Drug-induced headache is usually described as dull, diffuse, and band-like, and usually starts in the early morning. The original headache (migraine or tension headache) has often been present for many years and the regular intake of drugs often started several years before people present. Patients take on average 30 or more tablets per week often containing several different substances. The drugs most often used are caffeine, ergotalkaloids, paracetamol, and pyrazolone derivates. Withdrawal is problematic as patients' symptoms take several weeks to resolve. However, chronic headache disappears or decreases by more than 50% in two-thirds of the patients. Positive predictors for successful treatment are migraine as primary headache, chronic headache lasting less than 10 years, and the regular intake of ergotamine. It is well worth considering if this might be the patient's problem before adding to it with more tablets!
4.4.5. How surgery can influence pain
It is interesting to note that a proportion of patients who mistakenly undergo surgery for non-sinogenic pain experience temporary relief from their symptoms, although their pain returns within a few weeks and nearly always within 9 months. It is hypothesised that the reason for a temporary or partial reduction in their pain is either the effect of cognitive dissonance or the effect of surgical trauma on the afferent fibres going to the trigeminal nucleus, which alters its threshold for spontaneous activity in the short term. In about a third of patients surgery does not significantly affect the pain and in a third the pain is made far worse (1284). Patients whose pain is made worse by surgery may develop a more unpleasant quality to the pain such as burning.The criteria for diagnosing chronic rhinosinusitis vary, but most studies quote more than three nasal symptoms for more than 3 months (490). It is important to note that the inclusion of facial pain/pressure "on its own does not constitute a suggestive history for rhinosinusitis in the absence of another major nasal symptom or sign" (1205). The evidence that a vacuum within a blocked sinus can causes protracted pain is poor. Transient facial pain in patients with other symptoms and signs of rhinosinusitis can occur with acute pressure changes when flying, diving or skiing but this resolves as the pressure within the sinuses equalises within hours through perfusion with the surrounding vasculature. Patients who repeatedly suffer these intermittent symptoms whilst there is a pressure change are often helped by surgery to open the ostia. Persistent blockage of the sinus ostia rarely causes continuous pain for example silent sinus syndrome that is due to a blocked sinus with resorbtion of its contents to the extent that the orbital floor prolapses into the maxillary sinus causes no pain (1285). Endoscopic sinus surgery (ESS) has been advocated by a few workers for facial pain in the absence of endoscopic or CT evidence of sinus disease or anatomical variations Cook et al. advocated ESS on patients with facial pain, which also occurred 'independently' of episodes of rhinosinusitis, with no CT evidence of sinus pathology (1286). Twelve of the 18 patients who underwent surgery in their series had a significant reduction in their pain severity, yet it is significant that, "complete elimination of symptoms was not accomplished in any patient". They had no evidence of ostiomeatal obstruction. If the cause of their pain was due to ostial obstruction then it might be anticipated that surgery would cure their symptoms of pain. This was not the case as they all had residual pain. Similarly Parsons et al. retrospectively described 34 patients with headaches who had contact points removed and found that whilst there was a 91% decrease in intensity and 84% decrease in frequency, 65% had persisting symptoms (1287). One possible reason for a temporary or partial reduction in their pain is either the effect of cognitive dissonance or the effect of surgical trauma on the afferent fibres going to the trigeminal nucleus and this might alter the nucleus and its threshold for spontaneous activity for up to several months as has been found when patients with midfacial segment pain undergo surgery (1174).
4.4.5.1. Post surgical pain/Neuropathic pain
Neuropathic pain arises as a direct consequence of a lesion or disease affecting the somatosensory system (1288). Neuropathic pain is often spontaneous or it can be an abnormal response to a non-painful stimulus. The pain is often deep, burning, gnawing, occasionally stabbing or like an electric shock. It may start after a relatively minor injury or surgical procedure. There may be an altered sensation in the area affected. Acquadro et al. noted that in those patients with preoperative pain who underwent endoscopic sinus surgery, 7% developed new pain, and 2% reported a worsening of their facial pain but none developed de novo pain if they had had no preoperative pain (1289). Indeed to date, there has been few reported cases of facial pain following ESS in previously painfree patients although it may be under-reported (1174, 1284). This fact is surprising given that open sinus surgery, in particular the Caldwell-Luc procedure has long been known to cause de novo facial pain. One study noted this complication in 46% of all patients who had undergone a Caldwell Luc procedure, including some who had no prior facial pain though this may be due to direct trauma to the infraorbital nerve (1290). Trauma causes pain that is mediated by myelinated A delta and unmyelinated C fibres. Prolonged stimulation of these can activate N-methyl-D-aspartate (NMDA) and cause central sensitisation. An alteration in central processing can then lead to an alteration in pain thresholds producing hyperalgesia or even lead to spontaneous firing of neurones and may produce reverberating circuits. It is also possible that antidromic flow in C fibres can cause the release of substance P or that efferent sympathetic flow can release noradrenaline; both these mechanisms have the potential to sensitise peripheral receptors (1291). Trauma can be an initiating factor by either altering the fibres within the trigeminal nucleus or by altering its somatosensory input, thereby altering nocioceptive fibres to or within the caudal nucleus of the trigeminal nerve. These mechanisms, by altering the neuroplasticity of the nerves to and within the trigeminal nucleus, result in neuropathic pain.
Amitriptyline has been shown to be effective in relieving post-traumatic neuralgia (1292) in doses of 75 mg or alternatively gabapentin or pregabalin (1284) (Evidence level III). Duloxetine may help, particularly if there is coexisting anxiety. These need to be given for 6 to 8 weeks before judging if they have helped. A local anaesthetic nerve block can sometimes be successful in blocking localised pain and having a more prolonged benefit. Lignocaine patches over the area can help as can transcutaneous electrical nerve stimulation. The management of patients with pain unresponsive to medical treatment should involve pain coping strategies that involve a pain management team and psychologist and cognitive behaviour therapy. Physical activity, treated depression and anxiety, as well as establishing work or other activities can play an important role. Opiates can help but care is needed in prescribing these as they can lead to tolerance and dependency, which is a further obstacle to recovery.
4.4.5.2. Contact points
The theories that implicate contact points within the nose as a cause of headache or facial pain originate from McAuliffe who described stimulating various points within the nasal cavity and paranasal sinuses in five individuals in whom he said that both touch and faradic current caused referred pain to areas of the face (1293). He illustrated this paper with diagrams that have been reproduced in many texts. These findings have been used to support the idea that mucosal contact points within the nasal cavity can cause referred pain, even though McAuliffe's studies did not describe contact point induced headache or facial pain (1294). McAuliffe's work has recently been repeated in a controlled study and was found not to produce the referred pain that he described (1295). The prevalence of a contact point has not only been found to be the same in an asymptomatic population as in a symptomatic population but, when a contact point was present in symptomatic patients with unilateral pain, it was found in the contralateral side to the pain in 50% (1296). Stammberger and Wolf postulated that variations in the anatomy of the nasal cavity result in mucus stasis, infection and ultimately facial pain (1297). They also stated that mucosal contact points might result in the release of the neurotransmitter peptide substance P, a recognised neurotransmitter in nociceptive fibres but there has been no in vitro or vivo work to substantiate this. For contact points to be credible as a cause of facial pain or headache they should also be a predictor of facial pain in the whole population (1298). Another observation is that nowhere else in the body does mucosa-mucosa contact cause pain.
Other authors have embraced these concepts to explain how pain might be induced by anatomical variants such as a concha bullosa (1299-1302), or a pneumatised superior turbinate touching the septum (1303). The description of the presence of anatomical 'abnormalities' such as a concha bullosa, a paradoxical middle turbinate, a superior turbinate touching the septum, or a large ethmoid bulla is a misnomer as these are variations that occur in asymptomatic populations. Case-controlled studies examining the prevalence of anatomical variations in patients with rhinosinusitis and asymptomatic control groups have shown no significant differences (277, 570, 571, 576, 578-580, 1177, 1199, 1303-1310). It seems probable that the majority of the case series in the literature that describe surgery for anatomical variations in patients with facial pain that responded to surgery, that is more often partial than complete and relatively short lived, result from the effect of cognitive dissonance (1311), or from surgery altering neuroplasticity within the brainstem sensory nuclear complex (1237, 1279, 1280, 1312). .
The IHS classification (1194) has entered mucosal contact point headache as a new entry but says the evidence for it is limited. "Controlled trials are recommended to validate it, using the selection criteria:
2. abolition of pain within 5 minutes after diagnostic topical application of local anaesthesia to the middle turbinate using placebo or other controls.
D. Pain resolves within 7 days, and does not recur, after surgical removal of mucosal contact points (abolition of pain is indicated by a score of zero on the visual analogue scale).
At present reports that support the removal of contact points are notable by their retrospective nature, the lack of any controlled study, ability to explain the prevalence of these findings in many asymptomatic people in the population (1313) and a failure to meet the criteria in the HIS classification (1194).
The theories that implicate contact points within the nose as a cause of headache or facial pain originate from McAuliffe who described stimulating various points within the nasal cavity and paranasal sinuses in five individuals in whom he said that both touch and faradic current caused referred pain to areas of the face (1293). He illustrated this paper with diagrams that have been reproduced in many texts. These findings have been used to support the idea that mucosal contact points within the nasal cavity can cause referred pain, even though McAuliffe's studies did not describe contact point induced headache or facial pain (1294). McAuliffe's work has recently been repeated in a controlled study and was found not to produce the referred pain that he described (1295). The prevalence of a contact point has not only been found to be the same in an asymptomatic population as in a symptomatic population but, when a contact point was present in symptomatic patients with unilateral pain, it was found in the contralateral side to the pain in 50% (1296). Stammberger and Wolf postulated that variations in the anatomy of the nasal cavity result in mucus stasis, infection and ultimately facial pain (1297). They also stated that mucosal contact points might result in the release of the neurotransmitter peptide substance P, a recognised neurotransmitter in nociceptive fibres but there has been no in vitro or vivo work to substantiate this. For contact points to be credible as a cause of facial pain or headache they should also be a predictor of facial pain in the whole population (1298). Another observation is that nowhere else in the body does mucosa-mucosa contact cause pain.
Other authors have embraced these concepts to explain how pain might be induced by anatomical variants such as a concha bullosa (1299-1302), or a pneumatised superior turbinate touching the septum (1303). The description of the presence of anatomical 'abnormalities' such as a concha bullosa, a paradoxical middle turbinate, a superior turbinate touching the septum, or a large ethmoid bulla is a misnomer as these are variations that occur in asymptomatic populations. Case-controlled studies examining the prevalence of anatomical variations in patients with rhinosinusitis and asymptomatic control groups have shown no significant differences (277, 570, 571, 576, 578-580, 1177, 1199, 1303-1310). It seems probable that the majority of the case series in the literature that describe surgery for anatomical variations in patients with facial pain that responded to surgery, that is more often partial than complete and relatively short lived, result from the effect of cognitive dissonance (1311), or from surgery altering neuroplasticity within the brainstem sensory nuclear complex (1237, 1279, 1280, 1312). .
The IHS classification (1194) has entered mucosal contact point headache as a new entry but says the evidence for it is limited. "Controlled trials are recommended to validate it, using the selection criteria:
- A. Intermittent pain localised to the periorbital or medial canthal or temporozygomatic regions fulfilling criteria C and D.
-
B. Linical, nasal endoscopic and/or CT imaging evidence of
mucosal contact points without acute sinusitis.
-
C. Evidence that the pain can be attributed to mucosal
contact based on at least one of the following:
2. abolition of pain within 5 minutes after diagnostic topical application of local anaesthesia to the middle turbinate using placebo or other controls.
D. Pain resolves within 7 days, and does not recur, after surgical removal of mucosal contact points (abolition of pain is indicated by a score of zero on the visual analogue scale).
At present reports that support the removal of contact points are notable by their retrospective nature, the lack of any controlled study, ability to explain the prevalence of these findings in many asymptomatic people in the population (1313) and a failure to meet the criteria in the HIS classification (1194).
4.4.6. Specific neurological conditions
4.4.6.1. Trigeminal neuralgia
The characteristic presentation of trigeminal neuralgia with paroxysms of severe lanciolating pain induced by a specific trigger point is well recognised. In more than one third of sufferers the pain occurs in both the maxillary and mandibular divisions, while in one fifth it is confined to the maxillary division. In a small number of patients only the ophthalmic division is affected (3%). Typical trigger points are the lips and naso-labial folds, but pain may also be triggered by touching the gingivae. A flush may be seen over the face but there are no sensory disturbances in primary trigeminal neuralgia. Remissions are common but the condition can also increase in severity. Younger patients should undergo MR imaging to exclude other pathology such as disseminating sclerosis that is identified in 2-4% of patients with trigeminal neuralgia. Tumours such as posterior fossa meningiomas or neuromas are found in 2% of patients presenting with trigeminal neuralgia reinforcing the need for imaging to exclude such pathology. Carbamazepine remains the first line medical treatment, with gabapentin, Lamotrigine (1314) (Evidence level IIb) and Topiramate (1315) (Evidence level IIb) being employed more frequentlyIn cases refractory to medical treatment, referral to specialist centres for consideration of other treatment modalities such as microvascular decompression or stereotactic radiotherapy may be appropriate.
4.4.6.2. Post-herpetic Neuralgia
This is pain following a herpes zoster infection, and is defined as pain recurring or continuing at the site of shingles after the onset of the rash. Up to 50% of elderly patients who have had shingles may develop post-herpetic neuralgia, though fortunately most recover during the first year. Antiviral agents help curtail the pain of acute shingles, and there is some evidence that they reduce the risk of subsequent postherpetic neuralgia. Various medical treatments may be helpful particularly carbamazepine or gabapentin with or without a tricyclic antidepressants (1316) (Category of evidence IV).
4.4.6.3. Atypical Facial Pain
This is very much a diagnosis of exclusion and care must be taken in reaching this conclusion, even when the patient has received previous opinions and no pathology has been identified. The history is often vague and inconsistent with widespread pain extending from the face onto other areas of the head and neck. The pain may move from one part of the face to another between different consultations and other symptoms such as 'mucus moving' in the sinuses are often described. A number of patients have such completely fixed ideas about their condition that they will not be convinced otherwise whatever the weight of evidence to the contrary. Pain is often described in dramatic terms in conjunction with an excess of other unpleasant life events. Many of these patients have a history of other pain syndromes and their extensive records show minimal progress despite various medications. They have often undergone previous sinus or dental surgery and may be resentful about their treatment. It is not uncommon for such patients to give a history of nasal trauma. Many patients with atypical facial pain exhibit significant psychological disturbance or a history of depression and are unable to function normally as a result of their pain. Some project a pessimistic view of treatment, almost giving the impression they do not wish to be rid of the pain that plays such a central role in their lives. A comprehensive examination (including nasendoscopy) is essential and imaging such as MRI is advisable to exclude pathology before the patient is labelled as having atypical pain. The management of such patients is challenging and confrontation is nearly always counterproductive. A good starting point is to reassure the patient that you recognise that they have genuine pain and an empathetic consultation with an explanation should be conducted. Drug treatment revolves around a gradual build-up to the higher analgesic and antidepressant levels of amitriptyline (75-100 mgs) at night (1317) (Evidence level II). The second line treatment includes gabapentin and carbamazepine. Patients should sympathetically be made aware that psychological factors may play a role in their condition and referral to a clinical psychologist or psychiatrist may be helpful (1318) (Evidence level IV). Referral to a pain clinic is often appropriate.
4.4.6.4. Myofascial pain
Myofascial pain causes a widespread, poorly defined aching in the neck, jaw or ear. It is five times more common in women and worse when the patient is tired or stressed. Tender points may be found in the sternomastoid or trapezius muscles and initiating factors include malocclusion or poor deltopectoral posture. This syndrome overlaps to a large degree with temporomandibular joint dysfunction. Reassurance, local heat treatment, ultrasound and massage help.
4.4.6.5. Ophthalmological
Uncorrected optical refractive errors can cause headaches, but their importance is exaggerated. Visual acuity is tested ideally with a Snellen chart and if there is a refractive problem this can be overcome by testing vision through a pinhole. Disease involving the optic nerve results in reduced acuity and colour vision. Pain on ocular movement is suggestive of optic neuritis or scleritis. It is vital to recognise acute glaucoma, which may cause severe orbital pain and headache. The patient may see haloes around lights, and circumcorneal injection can occur as well as systemic upset, especially vomiting. This condition requires urgent treatment as vision is rapidly lost. Pain is a feature of periorbital cellulitis, which may present with lid swelling and erythema if it is preseptal and with chemosis, proptosis and reduced mobility if it arises posterior to the septum. Orbital pain can also be caused by uveitis, keratitis, dry eye syndrome and convergence insufficiency.Orbital haemorrhage can cause sudden pain, proptosis, nausea and vomiting, along with ecchymosis, reduced mobility and oedema of the optic disc. It may be secondary to an orbital varix, blood dyscrasias, hypertension or trauma.
The term 'Inflammatory Orbital Pseudotumour' should not be used for disorders whose aetiology is known (polyarteritis nodosa and vasculitis). This condition probably has an immunological basis is often a precursor of lymphona and it can produce pain, proptosis, reduced mobility, lid swelling and injection of the eye. Some individuals have recurring bouts and are pain free, whereas others have pain with upper respiratory tract infections and these can mistakenly be held responsible. The majority of other causes of proptosis are painless, such as hyperthyroidism and tumours of the orbit or of adjacent structures.
4.4.6.1. Trigeminal neuralgia
The characteristic presentation of trigeminal neuralgia with paroxysms of severe lanciolating pain induced by a specific trigger point is well recognised. In more than one third of sufferers the pain occurs in both the maxillary and mandibular divisions, while in one fifth it is confined to the maxillary division. In a small number of patients only the ophthalmic division is affected (3%). Typical trigger points are the lips and naso-labial folds, but pain may also be triggered by touching the gingivae. A flush may be seen over the face but there are no sensory disturbances in primary trigeminal neuralgia. Remissions are common but the condition can also increase in severity. Younger patients should undergo MR imaging to exclude other pathology such as disseminating sclerosis that is identified in 2-4% of patients with trigeminal neuralgia. Tumours such as posterior fossa meningiomas or neuromas are found in 2% of patients presenting with trigeminal neuralgia reinforcing the need for imaging to exclude such pathology. Carbamazepine remains the first line medical treatment, with gabapentin, Lamotrigine (1314) (Evidence level IIb) and Topiramate (1315) (Evidence level IIb) being employed more frequentlyIn cases refractory to medical treatment, referral to specialist centres for consideration of other treatment modalities such as microvascular decompression or stereotactic radiotherapy may be appropriate.
4.4.6.2. Post-herpetic Neuralgia
This is pain following a herpes zoster infection, and is defined as pain recurring or continuing at the site of shingles after the onset of the rash. Up to 50% of elderly patients who have had shingles may develop post-herpetic neuralgia, though fortunately most recover during the first year. Antiviral agents help curtail the pain of acute shingles, and there is some evidence that they reduce the risk of subsequent postherpetic neuralgia. Various medical treatments may be helpful particularly carbamazepine or gabapentin with or without a tricyclic antidepressants (1316) (Category of evidence IV).
4.4.6.3. Atypical Facial Pain
This is very much a diagnosis of exclusion and care must be taken in reaching this conclusion, even when the patient has received previous opinions and no pathology has been identified. The history is often vague and inconsistent with widespread pain extending from the face onto other areas of the head and neck. The pain may move from one part of the face to another between different consultations and other symptoms such as 'mucus moving' in the sinuses are often described. A number of patients have such completely fixed ideas about their condition that they will not be convinced otherwise whatever the weight of evidence to the contrary. Pain is often described in dramatic terms in conjunction with an excess of other unpleasant life events. Many of these patients have a history of other pain syndromes and their extensive records show minimal progress despite various medications. They have often undergone previous sinus or dental surgery and may be resentful about their treatment. It is not uncommon for such patients to give a history of nasal trauma. Many patients with atypical facial pain exhibit significant psychological disturbance or a history of depression and are unable to function normally as a result of their pain. Some project a pessimistic view of treatment, almost giving the impression they do not wish to be rid of the pain that plays such a central role in their lives. A comprehensive examination (including nasendoscopy) is essential and imaging such as MRI is advisable to exclude pathology before the patient is labelled as having atypical pain. The management of such patients is challenging and confrontation is nearly always counterproductive. A good starting point is to reassure the patient that you recognise that they have genuine pain and an empathetic consultation with an explanation should be conducted. Drug treatment revolves around a gradual build-up to the higher analgesic and antidepressant levels of amitriptyline (75-100 mgs) at night (1317) (Evidence level II). The second line treatment includes gabapentin and carbamazepine. Patients should sympathetically be made aware that psychological factors may play a role in their condition and referral to a clinical psychologist or psychiatrist may be helpful (1318) (Evidence level IV). Referral to a pain clinic is often appropriate.
4.4.6.4. Myofascial pain
Myofascial pain causes a widespread, poorly defined aching in the neck, jaw or ear. It is five times more common in women and worse when the patient is tired or stressed. Tender points may be found in the sternomastoid or trapezius muscles and initiating factors include malocclusion or poor deltopectoral posture. This syndrome overlaps to a large degree with temporomandibular joint dysfunction. Reassurance, local heat treatment, ultrasound and massage help.
4.4.6.5. Ophthalmological
Uncorrected optical refractive errors can cause headaches, but their importance is exaggerated. Visual acuity is tested ideally with a Snellen chart and if there is a refractive problem this can be overcome by testing vision through a pinhole. Disease involving the optic nerve results in reduced acuity and colour vision. Pain on ocular movement is suggestive of optic neuritis or scleritis. It is vital to recognise acute glaucoma, which may cause severe orbital pain and headache. The patient may see haloes around lights, and circumcorneal injection can occur as well as systemic upset, especially vomiting. This condition requires urgent treatment as vision is rapidly lost. Pain is a feature of periorbital cellulitis, which may present with lid swelling and erythema if it is preseptal and with chemosis, proptosis and reduced mobility if it arises posterior to the septum. Orbital pain can also be caused by uveitis, keratitis, dry eye syndrome and convergence insufficiency.Orbital haemorrhage can cause sudden pain, proptosis, nausea and vomiting, along with ecchymosis, reduced mobility and oedema of the optic disc. It may be secondary to an orbital varix, blood dyscrasias, hypertension or trauma.
The term 'Inflammatory Orbital Pseudotumour' should not be used for disorders whose aetiology is known (polyarteritis nodosa and vasculitis). This condition probably has an immunological basis is often a precursor of lymphona and it can produce pain, proptosis, reduced mobility, lid swelling and injection of the eye. Some individuals have recurring bouts and are pain free, whereas others have pain with upper respiratory tract infections and these can mistakenly be held responsible. The majority of other causes of proptosis are painless, such as hyperthyroidism and tumours of the orbit or of adjacent structures.
4.4.7. Conclusion
The key message in this evidence based review on facial pain is that contrary to the preconceptions of many patients and their primary care physicians, the majority of patients with facial pain or headache do not have rhinosinusitis. It is very important to ensure that the surgeon has the correct diagnosis in a patient with facial pain before embarking on any surgical treatment. Not only do the vast majority of patients with facial pain have a neurological cause, but in the small proportion that have paranasal sinus disease, the majority respond to medical treatment. We describe the prevalence and characteristics of the different causes of facial pain and headache and the symptoms and signs that are found in acute and chronic rhinosinusitis in order to help differentiate this group from other diagnoses.
The key message in this evidence based review on facial pain is that contrary to the preconceptions of many patients and their primary care physicians, the majority of patients with facial pain or headache do not have rhinosinusitis. It is very important to ensure that the surgeon has the correct diagnosis in a patient with facial pain before embarking on any surgical treatment. Not only do the vast majority of patients with facial pain have a neurological cause, but in the small proportion that have paranasal sinus disease, the majority respond to medical treatment. We describe the prevalence and characteristics of the different causes of facial pain and headache and the symptoms and signs that are found in acute and chronic rhinosinusitis in order to help differentiate this group from other diagnoses.
4.5. Genetics of CRS with and without nasal polyps
4.5.1. Summary
Chronic rhinosinusitis (CRS) is a complex disease, with a pathophysiology that is likely to be affected by multiple genetic and environmental factors. There are several studies that linked different chromosomal associations and single nucleotides polymorphism to CRS. Although genetic studies will probably not answer all questions, it should provide new information to redirect basic science studies to disease-related pathophysiological pathways. In the future we hope to be able to improve diagnostics and treatments for patients with CRS using the subclassification of the disease on the basis of genetics. Additionally, identification of environmental factors that may interact with subject's genome may also help to avoid these risk factors and potentially prevent disease expression.
Chronic rhinosinusitis (CRS) is a complex disease, with a pathophysiology that is likely to be affected by multiple genetic and environmental factors.
4.5.2. Introduction
Genetic studies to identify genes that could be responsible for certain disease can be performed with different techniques. These include candidate gene studies, linkage studies, or genome-wide association studies (GWAS). However, in genetic studies the GWA approach is rapidly replacing the more traditional candidate gene studies and microsatellite-based linkage mapping studies. The GWA approach would be useful to identify causal genes related to complex diseases such as CRS, due to its comprehensive and unbiased strategy. This progress was made possible by key developments in human genomics over the last decade and the completion of human genome DNA sequence analysis (HapMap) (1319). A molecular pathway based approach has been recently developed to facilitate more powerful analysis of GWA study data sets. In GWAS the basic principal is to compare the frequency of a genetic variant between cases and controls. Many genetic variants (SNPs) are tested (usually 300,000 to 1 million) and therefore adjustment for multiple testing is required. For example for NIH catalog of GWAS p values of 5x 10-8 or less are required (www.genome. gov/GWAS) (615). For any gene variant to be considered possibly significant for a given disease, it has to be replicated in at least two different, independent patient cohorts.
In addition to the direct effect of differences between genotypes we must also consider inter-individual and inter-tissue variations in gene transcript levels. These differences can be important in mediating disease susceptibility and may be caused by either genetic or epigenetic variation. Genetic variants influencing transcription include large-scale structural changes in the genome such as gene duplications and deletions; equally they can arise due to polymorphisms in a gene's regulatory elements.
4.5.3.Chronic rhinosinusitis with and without nasal polyps. (CRSw and CRSsNP)
4.5.3.1. Family and twin studies
An interesting observation is that chronic rhinosinusitis with nasal polyps (CRSwNP) is frequently found to run in families, suggesting a hereditary or with shared environmental factor. Alexiou et al. (1320) studied 100 patients with NP and 102 controls from the general population and showed that 13.3% of the patients and none of the controls had a history of polyps in the family. In the study by Rugina et al. (508), more than half of 224 CRSwNP patients (52%) had a positive family history of NP. The presence of CRSwNP was considered when NP had been diagnosed by an ENT practitioner or the patients had undergone sinus surgery for CRSwNP. A lower percentage (14%) of familial occurrence of CRSwNP was reported earlier by Greisner et al. (1321) in smaller group (n = 50) of adult patients with CRSwNP. Thus, these results strongly suggest the existence of a hereditary factor in the pathogenesis of CRSwNP. However, studies of monozygotic twins have not shown that both siblings always develop polyps, indicating that environmental factors are likely to influence the occurrence of NP (1322, 1323). CRSwNPs have been described in identical twins, but given the prevalence of nasal polyps it might be expected that there would be more than a rare report of this finding (1324).
Studies of monozygotic twins have not shown that both siblings always develop polyps, indicating that environmental factors are likely to influence the occurrence of CRSwNP
4.5.3.2. Linkage analysis and association studies
Most genetic studies of CRS to date are candidate gene or candidate pathway approach studies. The focus in these studies has been the role of innate immunity in the pathophysiology of CRS. Large-scale genome wide associations studies (GWAS) of CRS are still lacking. For GWAS to have sufficient statistical power, larger patient cohorts are needed than that have been used up to now. There is one study in CRS using a DNA poolbased GWA, a technique that was developed to reduce costs of GWAS by replacing individual DNA genotyping by pooled genomic DNA (1325). The technique uses separate pools of DNA from patients and controls and hybridizes these pools on highdensity single nucleotide polymorphisms (SNPs) microarrays to determine the allele frequencies in each pool (1326). In this study with 210 CRS patients and 189 controls, the authors identified 600 SNPs from 445 genes that were potentially associated with CRS. Authors stated that validation in a bigger cohort is needed to separate true positive results from the false positive.
Several affected genes and enriched SNPs have been published for patients with CRS with polyposis (CRSwNP) or without polyposis (CRSsNP). Three SNPs related to CRSwNP have been replicated this far and they are genes IL1A, TNF , and AOAH.
In the near future, due to the decreasing cost of GWAS technology, it is expected that large-scale GWAS studies of CRS will soon be performed as well.
Several affected genes and enriched SNPs have been published for patients with CRSw and sNP Three SNPs related to CRSwNP have been replicated this far. These are for the genes IL1α (1327, 1328). TNF (1329), and AOAH (1321, 1328). The original study of association of IL1α, IL1β and TNF in CRSwNP in a Turkish population was published 2007 (1330). Association of IL1A to the development of severe CRS was reported in a replication study of 206 patients (1328). Patients had had at least one endoscopic sinus surgery and their symptoms persisted. Nasal polyps was the initial diagnosis in 74,8% of the patients in this study. The TNFA association to nasal polyps was replicated in study of 170 CRSwNP patients compared to 153 non-polyposis controls (1329).
Several other polymorphisms associated with CRS have been published but have not been replicated, including polymorphism in IL-22 (1331), and the heterozygote status for the alpha-1-antitrypsin (AAT) gene (SERAPINA1) (1332) in severe CRSwNP, as well as for the IL-1 receptor-associated kinase 4 (IRAK4) (1333) and MET gene (1334) in a Canadian population. Two SNPs in Toll-like receptor 2 (TLR2) were associated with increased risk of CRS in the Korean population (1335) suggesting that these SNPs may affect the susceptibility to bacterial infections leading to development of CRS. In another study, polymorphism of IL-4 (IL-4/-590 C-T), a potential determinant of IgE mediated allergic disease, was found to be associated with a protective mechanism against NPs in the Korean populations (1336). The role of IL-33 pathway in development of pathogenesis of NP was studied in 284 NP-patients from Belgium. Thiss tudy found two SNPs in the IL-33 ILIRL1 pathway to increase susceptibility for NP (840).
Several other polymorphisms associated with CRS have been published but not been replicated.
A population based genome-wide screen for CRS among 291 Hutterites (isolated religious community in US and Canada) linked a locus on chromosome 7q to the disease, suggesting CFTR (cystic fibrosis transmembrane conductance regulator) gene influencing disease susceptibility (1337). Reduced expression of several epithelial genes, like S100A7, S100A8 and SPINK5 has been reported in CRSwNP and CRSsNP. These finding suggest alterations in epithelial barrier function and host defense in CRS (782).
A number of genetic association studies found a significant correlation between certain HLA (human leukocyte antigen) alleles and NP. HLA is the general name of a group of genes in the human major histocompatibility complex (MHC) region on the human chromosome 6 that encodes the cell-surface antigen-presenting proteins. Luxenberger et al. (1338) reported an association between HLA-A74 and NPs, whereas Molnar-Gabor et al. (1339) reported that subjects carrying HLA-DR7-DQA1*0201 and HLA-DR7¬DQB1*0202 haplotype had a 2 to 3 times odds ratio of developing NP. The risk of developing NP can be as high as 5.53 times in subjects with HLA¬DQA1*0201-DQB1*0201 haplotype (1340). Although several HLA alleles were found to be associated with NP, such susceptibility can be influenced by ethnicity. In the Mexican Mestizo population, increased frequency of the HLA¬DRB1*03 allele and of the HLA-DRB1*04 allele were found in patients with NP as compared to healthy controls (1341). Fruth et al. (1342) studied Glutathione S-Transferases (GST) as one of major group of antioxidative active enzymes involved in cellular detoxification. The authors analyzed 170 nasal tissue samples (CRS without nasal polyps=49, CRS with nasal polyps=69 and healthy tissue controls of the inferior turbinate=52) and concluded that there is no correlation between any GST-polymorphism and CRS with and without nasal polyps or allergies or asthma or aspirin-intolerance.
4.5.1. Summary
Chronic rhinosinusitis (CRS) is a complex disease, with a pathophysiology that is likely to be affected by multiple genetic and environmental factors. There are several studies that linked different chromosomal associations and single nucleotides polymorphism to CRS. Although genetic studies will probably not answer all questions, it should provide new information to redirect basic science studies to disease-related pathophysiological pathways. In the future we hope to be able to improve diagnostics and treatments for patients with CRS using the subclassification of the disease on the basis of genetics. Additionally, identification of environmental factors that may interact with subject's genome may also help to avoid these risk factors and potentially prevent disease expression.
Chronic rhinosinusitis (CRS) is a complex disease, with a pathophysiology that is likely to be affected by multiple genetic and environmental factors.
4.5.2. Introduction
Genetic studies to identify genes that could be responsible for certain disease can be performed with different techniques. These include candidate gene studies, linkage studies, or genome-wide association studies (GWAS). However, in genetic studies the GWA approach is rapidly replacing the more traditional candidate gene studies and microsatellite-based linkage mapping studies. The GWA approach would be useful to identify causal genes related to complex diseases such as CRS, due to its comprehensive and unbiased strategy. This progress was made possible by key developments in human genomics over the last decade and the completion of human genome DNA sequence analysis (HapMap) (1319). A molecular pathway based approach has been recently developed to facilitate more powerful analysis of GWA study data sets. In GWAS the basic principal is to compare the frequency of a genetic variant between cases and controls. Many genetic variants (SNPs) are tested (usually 300,000 to 1 million) and therefore adjustment for multiple testing is required. For example for NIH catalog of GWAS p values of 5x 10-8 or less are required (www.genome. gov/GWAS) (615). For any gene variant to be considered possibly significant for a given disease, it has to be replicated in at least two different, independent patient cohorts.
In addition to the direct effect of differences between genotypes we must also consider inter-individual and inter-tissue variations in gene transcript levels. These differences can be important in mediating disease susceptibility and may be caused by either genetic or epigenetic variation. Genetic variants influencing transcription include large-scale structural changes in the genome such as gene duplications and deletions; equally they can arise due to polymorphisms in a gene's regulatory elements.
4.5.3.Chronic rhinosinusitis with and without nasal polyps. (CRSw and CRSsNP)
4.5.3.1. Family and twin studies
An interesting observation is that chronic rhinosinusitis with nasal polyps (CRSwNP) is frequently found to run in families, suggesting a hereditary or with shared environmental factor. Alexiou et al. (1320) studied 100 patients with NP and 102 controls from the general population and showed that 13.3% of the patients and none of the controls had a history of polyps in the family. In the study by Rugina et al. (508), more than half of 224 CRSwNP patients (52%) had a positive family history of NP. The presence of CRSwNP was considered when NP had been diagnosed by an ENT practitioner or the patients had undergone sinus surgery for CRSwNP. A lower percentage (14%) of familial occurrence of CRSwNP was reported earlier by Greisner et al. (1321) in smaller group (n = 50) of adult patients with CRSwNP. Thus, these results strongly suggest the existence of a hereditary factor in the pathogenesis of CRSwNP. However, studies of monozygotic twins have not shown that both siblings always develop polyps, indicating that environmental factors are likely to influence the occurrence of NP (1322, 1323). CRSwNPs have been described in identical twins, but given the prevalence of nasal polyps it might be expected that there would be more than a rare report of this finding (1324).
Studies of monozygotic twins have not shown that both siblings always develop polyps, indicating that environmental factors are likely to influence the occurrence of CRSwNP
4.5.3.2. Linkage analysis and association studies
Most genetic studies of CRS to date are candidate gene or candidate pathway approach studies. The focus in these studies has been the role of innate immunity in the pathophysiology of CRS. Large-scale genome wide associations studies (GWAS) of CRS are still lacking. For GWAS to have sufficient statistical power, larger patient cohorts are needed than that have been used up to now. There is one study in CRS using a DNA poolbased GWA, a technique that was developed to reduce costs of GWAS by replacing individual DNA genotyping by pooled genomic DNA (1325). The technique uses separate pools of DNA from patients and controls and hybridizes these pools on highdensity single nucleotide polymorphisms (SNPs) microarrays to determine the allele frequencies in each pool (1326). In this study with 210 CRS patients and 189 controls, the authors identified 600 SNPs from 445 genes that were potentially associated with CRS. Authors stated that validation in a bigger cohort is needed to separate true positive results from the false positive.
Several affected genes and enriched SNPs have been published for patients with CRS with polyposis (CRSwNP) or without polyposis (CRSsNP). Three SNPs related to CRSwNP have been replicated this far and they are genes IL1A, TNF , and AOAH.
In the near future, due to the decreasing cost of GWAS technology, it is expected that large-scale GWAS studies of CRS will soon be performed as well.
Several affected genes and enriched SNPs have been published for patients with CRSw and sNP Three SNPs related to CRSwNP have been replicated this far. These are for the genes IL1α (1327, 1328). TNF (1329), and AOAH (1321, 1328). The original study of association of IL1α, IL1β and TNF in CRSwNP in a Turkish population was published 2007 (1330). Association of IL1A to the development of severe CRS was reported in a replication study of 206 patients (1328). Patients had had at least one endoscopic sinus surgery and their symptoms persisted. Nasal polyps was the initial diagnosis in 74,8% of the patients in this study. The TNFA association to nasal polyps was replicated in study of 170 CRSwNP patients compared to 153 non-polyposis controls (1329).
Several other polymorphisms associated with CRS have been published but have not been replicated, including polymorphism in IL-22 (1331), and the heterozygote status for the alpha-1-antitrypsin (AAT) gene (SERAPINA1) (1332) in severe CRSwNP, as well as for the IL-1 receptor-associated kinase 4 (IRAK4) (1333) and MET gene (1334) in a Canadian population. Two SNPs in Toll-like receptor 2 (TLR2) were associated with increased risk of CRS in the Korean population (1335) suggesting that these SNPs may affect the susceptibility to bacterial infections leading to development of CRS. In another study, polymorphism of IL-4 (IL-4/-590 C-T), a potential determinant of IgE mediated allergic disease, was found to be associated with a protective mechanism against NPs in the Korean populations (1336). The role of IL-33 pathway in development of pathogenesis of NP was studied in 284 NP-patients from Belgium. Thiss tudy found two SNPs in the IL-33 ILIRL1 pathway to increase susceptibility for NP (840).
Several other polymorphisms associated with CRS have been published but not been replicated.
A population based genome-wide screen for CRS among 291 Hutterites (isolated religious community in US and Canada) linked a locus on chromosome 7q to the disease, suggesting CFTR (cystic fibrosis transmembrane conductance regulator) gene influencing disease susceptibility (1337). Reduced expression of several epithelial genes, like S100A7, S100A8 and SPINK5 has been reported in CRSwNP and CRSsNP. These finding suggest alterations in epithelial barrier function and host defense in CRS (782).
A number of genetic association studies found a significant correlation between certain HLA (human leukocyte antigen) alleles and NP. HLA is the general name of a group of genes in the human major histocompatibility complex (MHC) region on the human chromosome 6 that encodes the cell-surface antigen-presenting proteins. Luxenberger et al. (1338) reported an association between HLA-A74 and NPs, whereas Molnar-Gabor et al. (1339) reported that subjects carrying HLA-DR7-DQA1*0201 and HLA-DR7¬DQB1*0202 haplotype had a 2 to 3 times odds ratio of developing NP. The risk of developing NP can be as high as 5.53 times in subjects with HLA¬DQA1*0201-DQB1*0201 haplotype (1340). Although several HLA alleles were found to be associated with NP, such susceptibility can be influenced by ethnicity. In the Mexican Mestizo population, increased frequency of the HLA¬DRB1*03 allele and of the HLA-DRB1*04 allele were found in patients with NP as compared to healthy controls (1341). Fruth et al. (1342) studied Glutathione S-Transferases (GST) as one of major group of antioxidative active enzymes involved in cellular detoxification. The authors analyzed 170 nasal tissue samples (CRS without nasal polyps=49, CRS with nasal polyps=69 and healthy tissue controls of the inferior turbinate=52) and concluded that there is no correlation between any GST-polymorphism and CRS with and without nasal polyps or allergies or asthma or aspirin-intolerance.
4.5.3.3. Multiple gene expressions in nasal polyps
No single gene has been shown to be uniquely related to CRS.
The development and persistence of mucosal inflammation in NPs have been reported to be associated with numerous genes and potential single nucleotide polymorphisms (SNPs). The products of these genes determine various disease processes, such as immune modulation or immuno-pathogenesis, inflammatory cells (e.g., lymphocytes, eosinophils, neutrophils) development, activation, migration and life span, adhesion molecule expression, cytokine synthesis, cell-surface receptor display, and processes governing fibrosis and epithelial remodelling. In the literature, gene expression profiles in nasal polyp have been performed by many studies, including the major repertoire of disease-related susceptibility genes or genotypic markers. With the advance of microassay technique, expression profiles of over 10,000 of known and novel genes can be detected. A recent study showed that in NP tissues, 192 genes were upregulated by at least 2-fold, and 156 genes were downregulated by at least 50% in NP tissues as compared to sphenoid sinuses mucosa (1059). In another study (1065), microarray analysis was used to investigate the expression profile of 491 immune-associated genes in nasal polyps. The results showed that 87 genes were differentially expressed in the immuneassociated gene profile of nasal polyps, and 15 genes showed differential expression in both NP and controls (turbinate). These seemingly conflicting results are likely due to the heterogeneity of inflammatory cells within nasal polyps and the differences in study designs and analytic approaches. In addition, in most of the published studies, the functional significance of aberrant gene expression with respective to the pathogenesis of NP is yet to be determined. The expression of gene products is regulated at multiple levels, such as during transcription, mRNA processing, translation, phosphorylation and degradation. Although some studies were able to show certain NP associated polymorphisms and genotypes, the present data is still fragmented. In common with many common human diseases, inherited genetic variation appears to be critical but yet still largely unexplained. Future studies are needed to identify the key genes underlying the development or formation of NP and to investigate the interactions between genetic and environmental factors that influence the complex traits of this disease. Identifying the causal genes and variants in NP is important in the path towards improved prevention, diagnosis and treatment of NPs.
A subset of CRSwNp patients has Samter's triad (ASA) characterized by presence of aspirin sensitivity, CRSwNP and asthma. Five different genes were reported to be associated in this group of 30 patients. The gene most characteristic of the ASA phenotype was periostin (POSTN) that was upregulated compared to controls. Also the proto-oncogene MET and protein phosphate 1 regulatory subunit (PP1R9B) were upregulated, whereas prolactin induced protein (PIP) and zinc alpha2 glycoprotein (Azgp1) were down-regulated (1029). A PubMed literature research (Jan1950-July 2010) was performed to identify candidate molecular markers associated with CRSwNP by Platt et al. (1343). Pathway analysis of molecular markers in CRSwNP included 554 genes that had fold change more than 3 and False Discovery Rate of less than 0.1 selected from the group's previous genome-wide expression study (1029). From these genes 365 were up-regulated and 189 were downregulated. The most common affected pathways for these genes were: inflammatory response, cellular movement, hematological system development and function, immune cell trafficking, and respiratory disease pathways. Gene network pathway analysis generated from the literature of this data showed tumor necrosis factor (TNF) as a central nodal molecule of the highest scoring network (p = 1 x 10-41) related to these pathways.
4.5.3.4. CRS and cystic fibrosis
The role of genetic factors in CRS has been implicated in patients with cystic fibrosis (CF) and primary ciliary dyskinesia (Kartagener's syndrome). CF is one of the most frequent autosomal recessive disorders of the Caucasian population, caused by mutations of the CFTR gene on chromosome 7 (564). The most common mutation, F508, is found in 70 to 80% of all CFTR genes in Northern Europe (1344, 1345). Upper airway manifestations of CF patients include CRS and nasal polyps, which are found in 25 to 40 % of CF patients above the age of 5 (1346-1349). Interestingly, Jorissen et al. (1350) reported that F508 homozygosity represents a risk factor for paranasal sinus disease in CF and Wang reported that mutations in the gene responsible for CF may be associated with the development of CRS in the general population (1351).
Conclusion
No single gene has been shown to be uniquely related to CRS. This is unlikely to change in the future due to a complexity of the disease and its pathophysiology. Only when CRS can be phenotyped into subgroups with similar pathophysiological features could we hope to detect the genes behind these subgroups more accurately
No single gene has been shown to be uniquely related to CRS.
The development and persistence of mucosal inflammation in NPs have been reported to be associated with numerous genes and potential single nucleotide polymorphisms (SNPs). The products of these genes determine various disease processes, such as immune modulation or immuno-pathogenesis, inflammatory cells (e.g., lymphocytes, eosinophils, neutrophils) development, activation, migration and life span, adhesion molecule expression, cytokine synthesis, cell-surface receptor display, and processes governing fibrosis and epithelial remodelling. In the literature, gene expression profiles in nasal polyp have been performed by many studies, including the major repertoire of disease-related susceptibility genes or genotypic markers. With the advance of microassay technique, expression profiles of over 10,000 of known and novel genes can be detected. A recent study showed that in NP tissues, 192 genes were upregulated by at least 2-fold, and 156 genes were downregulated by at least 50% in NP tissues as compared to sphenoid sinuses mucosa (1059). In another study (1065), microarray analysis was used to investigate the expression profile of 491 immune-associated genes in nasal polyps. The results showed that 87 genes were differentially expressed in the immuneassociated gene profile of nasal polyps, and 15 genes showed differential expression in both NP and controls (turbinate). These seemingly conflicting results are likely due to the heterogeneity of inflammatory cells within nasal polyps and the differences in study designs and analytic approaches. In addition, in most of the published studies, the functional significance of aberrant gene expression with respective to the pathogenesis of NP is yet to be determined. The expression of gene products is regulated at multiple levels, such as during transcription, mRNA processing, translation, phosphorylation and degradation. Although some studies were able to show certain NP associated polymorphisms and genotypes, the present data is still fragmented. In common with many common human diseases, inherited genetic variation appears to be critical but yet still largely unexplained. Future studies are needed to identify the key genes underlying the development or formation of NP and to investigate the interactions between genetic and environmental factors that influence the complex traits of this disease. Identifying the causal genes and variants in NP is important in the path towards improved prevention, diagnosis and treatment of NPs.
A subset of CRSwNp patients has Samter's triad (ASA) characterized by presence of aspirin sensitivity, CRSwNP and asthma. Five different genes were reported to be associated in this group of 30 patients. The gene most characteristic of the ASA phenotype was periostin (POSTN) that was upregulated compared to controls. Also the proto-oncogene MET and protein phosphate 1 regulatory subunit (PP1R9B) were upregulated, whereas prolactin induced protein (PIP) and zinc alpha2 glycoprotein (Azgp1) were down-regulated (1029). A PubMed literature research (Jan1950-July 2010) was performed to identify candidate molecular markers associated with CRSwNP by Platt et al. (1343). Pathway analysis of molecular markers in CRSwNP included 554 genes that had fold change more than 3 and False Discovery Rate of less than 0.1 selected from the group's previous genome-wide expression study (1029). From these genes 365 were up-regulated and 189 were downregulated. The most common affected pathways for these genes were: inflammatory response, cellular movement, hematological system development and function, immune cell trafficking, and respiratory disease pathways. Gene network pathway analysis generated from the literature of this data showed tumor necrosis factor (TNF) as a central nodal molecule of the highest scoring network (p = 1 x 10-41) related to these pathways.
4.5.3.4. CRS and cystic fibrosis
The role of genetic factors in CRS has been implicated in patients with cystic fibrosis (CF) and primary ciliary dyskinesia (Kartagener's syndrome). CF is one of the most frequent autosomal recessive disorders of the Caucasian population, caused by mutations of the CFTR gene on chromosome 7 (564). The most common mutation, F508, is found in 70 to 80% of all CFTR genes in Northern Europe (1344, 1345). Upper airway manifestations of CF patients include CRS and nasal polyps, which are found in 25 to 40 % of CF patients above the age of 5 (1346-1349). Interestingly, Jorissen et al. (1350) reported that F508 homozygosity represents a risk factor for paranasal sinus disease in CF and Wang reported that mutations in the gene responsible for CF may be associated with the development of CRS in the general population (1351).
Conclusion
No single gene has been shown to be uniquely related to CRS. This is unlikely to change in the future due to a complexity of the disease and its pathophysiology. Only when CRS can be phenotyped into subgroups with similar pathophysiological features could we hope to detect the genes behind these subgroups more accurately